Abstract
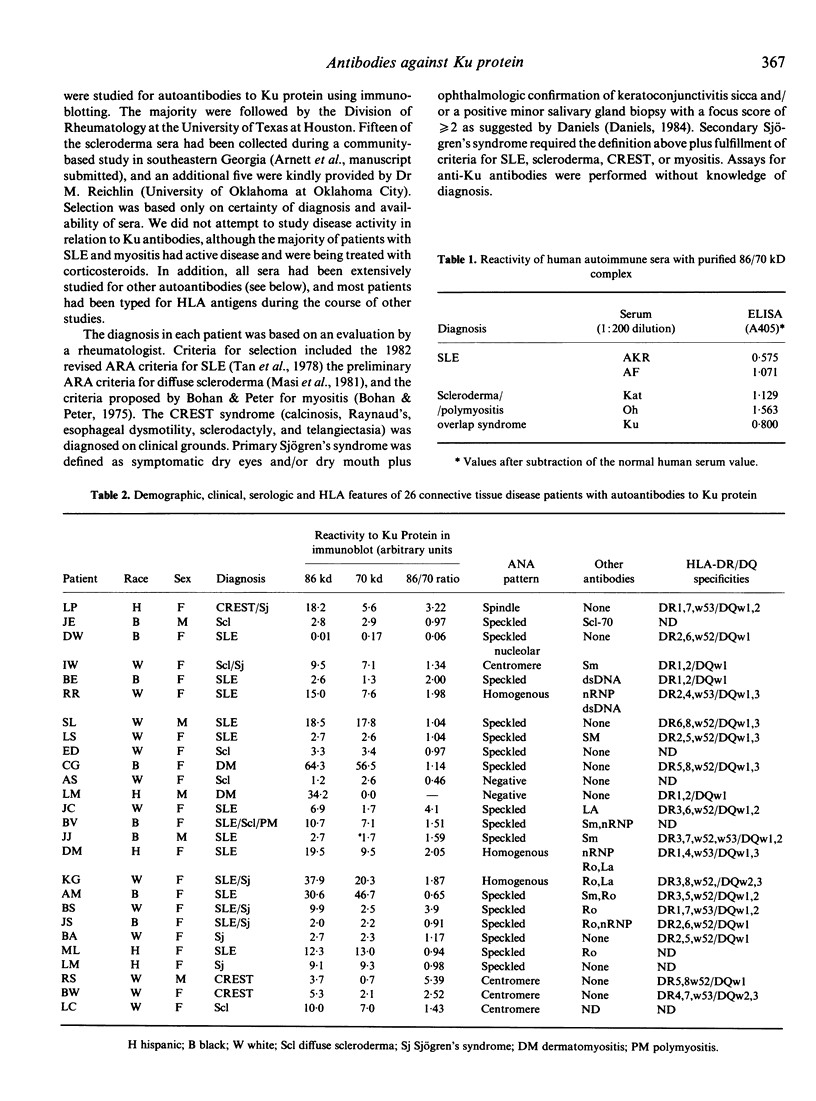
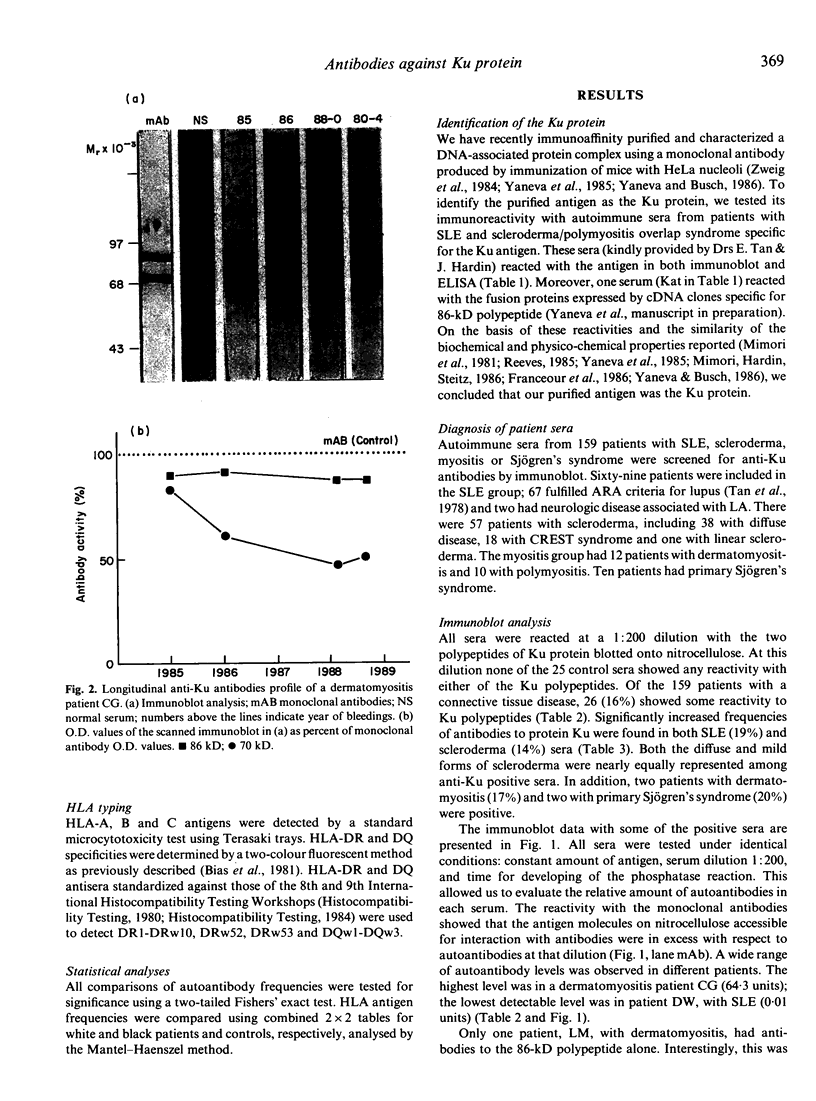
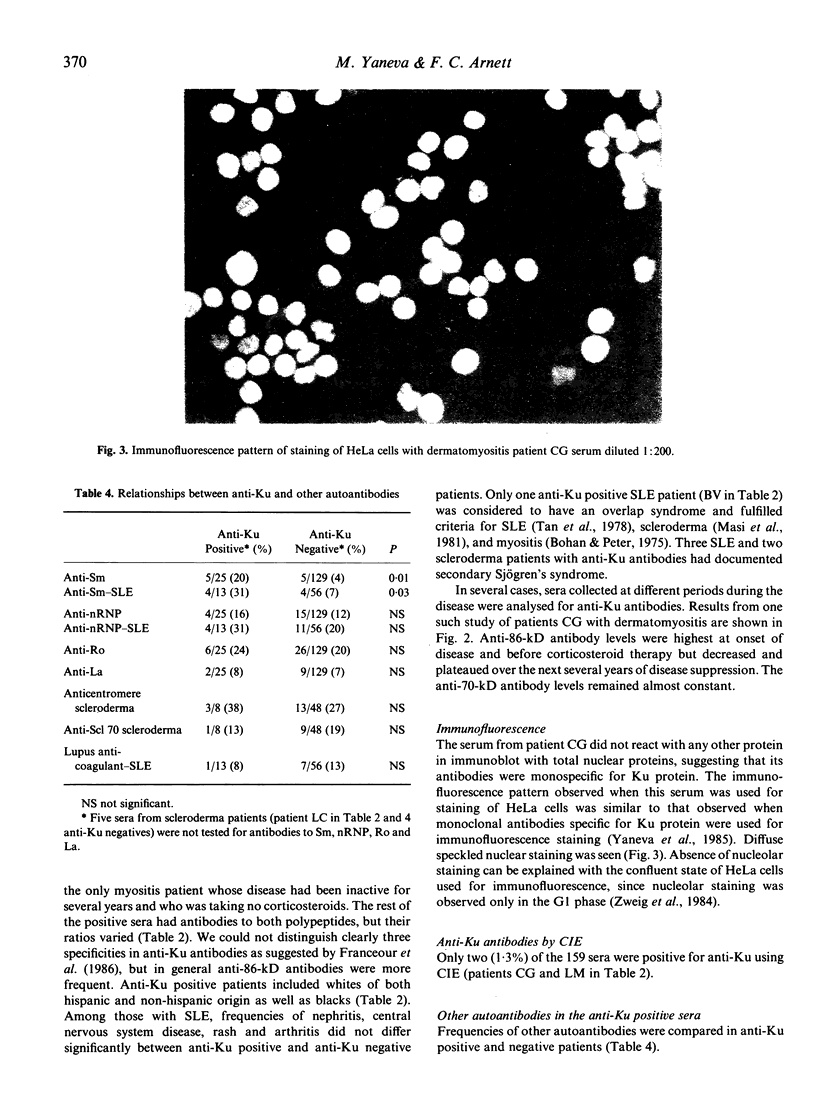
Immunoaffinity-purified Ku protein was used to screen sera from patients with systemic lupus erythematosus (SLE), scleroderma, myositis and Sjögren's syndrome for anti-Ku antibodies in a quantitative immunoblot assay. Sixteen percent of the 159 studied sera were reactive with the Ku protein; significantly increased frequencies of anti-Ku antibodies were found in SLE (19%) and scleroderma (14%) sera. Patients with myositis and Sjögren's syndrome showed similar frequencies. All positive sera had antibodies to the 86 kD subunit of Ku protein; only one serum did not react with 70 kD subunit. Frequencies of other autoantibodies were compared in anti-Ku positive and negative patients. Only anti-Sm antibodies, especially in the absence of anti-nRNP, appear to be associated with the presence of anti-Ku antibodies. A strong correlation between anti-Ku antibodies and the class II HLA antigen DQw1 (89% of the positive sera) was observed, suggesting participation of MHC genes in the mounting of the anti-Ku immune response.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnett F. C., Hamilton R. G., Roebber M. G., Harley J. B., Reichlin M. Increased frequencies of Sm and nRNP autoantibodies in American blacks compared to whites with systemic lupus erythematosus. J Rheumatol. 1988 Dec;15(12):1773–1776. [PubMed] [Google Scholar]
- Bias W. B., Hsu S. H., Pollard M. K., Harvey J., Lotze M. T., Arnett F. C., Stevens M. B. HLA-DR characterization of a Chippewa Indian subpopulation with high prevalence of rheumatoid arthritis. Hum Immunol. 1981 Mar;2(2):155–163. doi: 10.1016/0198-8859(81)90062-8. [DOI] [PubMed] [Google Scholar]
- Bohan A., Peter J. B. Polymyositis and dermatomyositis (first of two parts). N Engl J Med. 1975 Feb 13;292(7):344–347. doi: 10.1056/NEJM197502132920706. [DOI] [PubMed] [Google Scholar]
- Craft J. E., Hardin J. A. Linked sets of antinuclear antibodies: what do they mean? J Rheumatol Suppl. 1987 Jun;14 (Suppl 13):106–109. [PubMed] [Google Scholar]
- Daniels T. E. Labial salivary gland biopsy in Sjögren's syndrome. Assessment as a diagnostic criterion in 362 suspected cases. Arthritis Rheum. 1984 Feb;27(2):147–156. doi: 10.1002/art.1780270205. [DOI] [PubMed] [Google Scholar]
- Engvall E. Enzyme immunoassay ELISA and EMIT. Methods Enzymol. 1980;70(A):419–439. doi: 10.1016/s0076-6879(80)70067-8. [DOI] [PubMed] [Google Scholar]
- Francoeur A. M., Peebles C. L., Gompper P. T., Tan E. M. Identification of Ki (Ku, p70/p80) autoantigens and analysis of anti-Ki autoantibody reactivity. J Immunol. 1986 Mar 1;136(5):1648–1653. [PubMed] [Google Scholar]
- Goldstein R., Arnett F. C. The genetics of rheumatic disease in man. Rheum Dis Clin North Am. 1987 Dec;13(3):487–510. [PubMed] [Google Scholar]
- Hamilton R. G., Harley J. B., Bias W. B., Roebber M., Reichlin M., Hochberg M. C., Arnett F. C. Two Ro (SS-A) autoantibody responses in systemic lupus erythematosus. Correlation of HLA-DR/DQ specificities with quantitative expression of Ro (SS-A) autoantibody. Arthritis Rheum. 1988 Apr;31(4):496–505. doi: 10.1002/art.1780310406. [DOI] [PubMed] [Google Scholar]
- Hardin J. A. The lupus autoantigens and the pathogenesis of systemic lupus erythematosus. Arthritis Rheum. 1986 Apr;29(4):457–460. doi: 10.1002/art.1780290401. [DOI] [PubMed] [Google Scholar]
- Johnson G. D., Edmonds J. P., Holborow E. J. Precipitating antibody to D.N.A. detected by two-stage electroimmunodiffusion. Study in S.L.E. and in rheumatoid arthritis. Lancet. 1973 Oct 20;2(7834):883–885. doi: 10.1016/s0140-6736(73)92008-4. [DOI] [PubMed] [Google Scholar]
- Maul G. G., French B. T., van Venrooij W. J., Jimenez S. A. Topoisomerase I identified by scleroderma 70 antisera: enrichment of topoisomerase I at the centromere in mouse mitotic cells before anaphase. Proc Natl Acad Sci U S A. 1986 Jul;83(14):5145–5149. doi: 10.1073/pnas.83.14.5145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarty G. A., Valencia D. W., Fritzler M. J. Antibody to the mitotic spindle apparatus: immunologic characteristics and cytologic studies. J Rheumatol. 1984 Apr;11(2):213–218. [PubMed] [Google Scholar]
- Mimori T., Akizuki M., Yamagata H., Inada S., Yoshida S., Homma M. Characterization of a high molecular weight acidic nuclear protein recognized by autoantibodies in sera from patients with polymyositis-scleroderma overlap. J Clin Invest. 1981 Sep;68(3):611–620. doi: 10.1172/JCI110295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mimori T., Hardin J. A., Steitz J. A. Characterization of the DNA-binding protein antigen Ku recognized by autoantibodies from patients with rheumatic disorders. J Biol Chem. 1986 Feb 15;261(5):2274–2278. [PubMed] [Google Scholar]
- Moroi Y., Peebles C., Fritzler M. J., Steigerwald J., Tan E. M. Autoantibody to centromere (kinetochore) in scleroderma sera. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1627–1631. doi: 10.1073/pnas.77.3.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves W. H. Use of monoclonal antibodies for the characterization of novel DNA-binding proteins recognized by human autoimmune sera. J Exp Med. 1985 Jan 1;161(1):18–39. doi: 10.1084/jem.161.1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan E. M., Cohen A. S., Fries J. F., Masi A. T., McShane D. J., Rothfield N. F., Schaller J. G., Talal N., Winchester R. J. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982 Nov;25(11):1271–1277. doi: 10.1002/art.1780251101. [DOI] [PubMed] [Google Scholar]
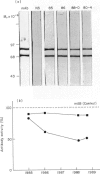
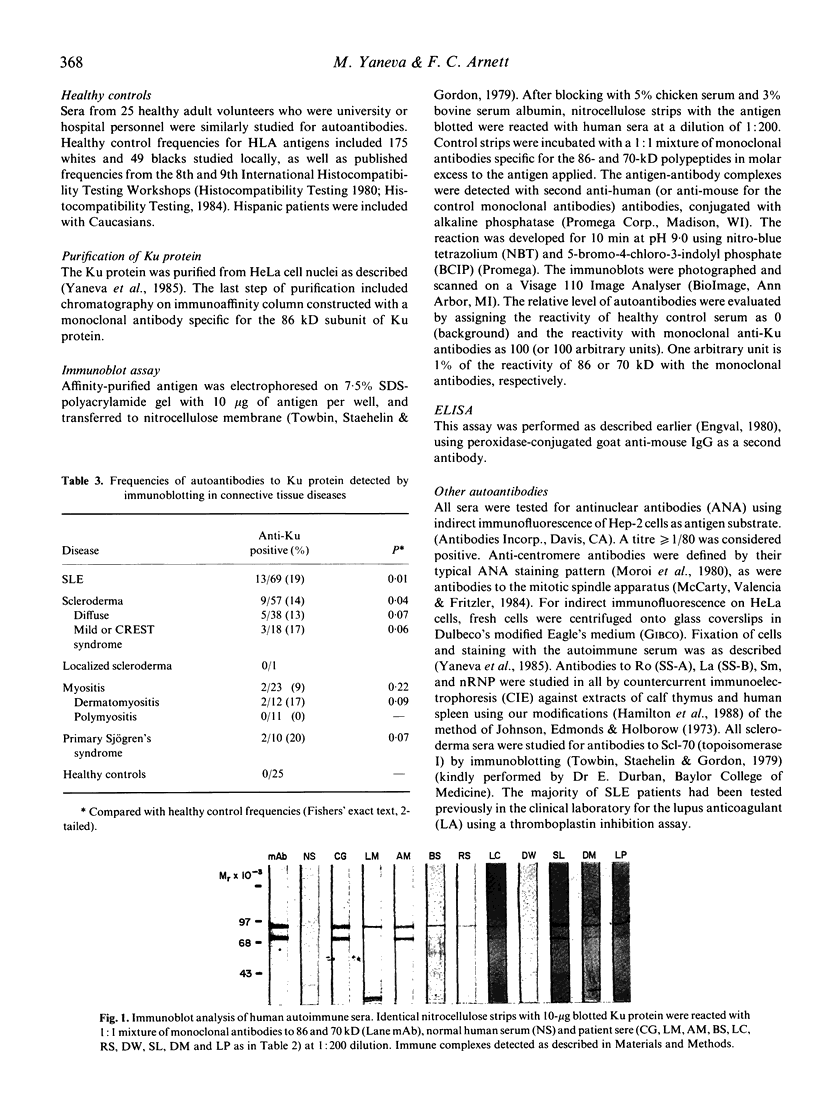
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaneva M., Busch H. A 10S particle released from deoxyribonuclease-sensitive regions of HeLa cell nuclei contains the 86-kilodalton-70-kilodalton protein complex. Biochemistry. 1986 Sep 9;25(18):5057–5063. doi: 10.1021/bi00366a013. [DOI] [PubMed] [Google Scholar]
- Yaneva M., Ochs R., McRorie D. K., Zweig S., Busch H. Purification of an 86-70 kDa nuclear DNA-associated protein complex. Biochim Biophys Acta. 1985 Jul 26;841(1):22–29. doi: 10.1016/0304-4165(85)90270-3. [DOI] [PubMed] [Google Scholar]
- van Venrooij W. J., Stapel S. O., Houben H., Habets W. J., Kallenberg C. G., Penner E., van de Putte L. B. Scl-86, a marker antigen for diffuse scleroderma. J Clin Invest. 1985 Mar;75(3):1053–1060. doi: 10.1172/JCI111767. [DOI] [PMC free article] [PubMed] [Google Scholar]