Abstract
We have compared the kinetics of antibody responses in conventional and dendritic cell-targeted immunization by using a model antigen in mice. Targeting was achieved by linking the reporter antigen (polyclonal goat anti-hamster antibody) to N418, a hamster mAb that binds to the CD11c molecule on the surface of murine dendritic cells. Intradermal injection of submicrogram quantities of goat anti-hamster antibody complexed to mAb N418 elicited goat antibody-specific serum IgG in mice. Antigen-specific IgG titers were detectable by day 5, with titers that ranged from 1:1000 to 1:100,000 by day 7. In contrast, when the goat antigen was injected alone or in the presence of a hamster antibody control to form nontargeted complexes, goat-specific serum IgG was undetectable at day 7. Additional control experiments showed that the interaction between the model antigen and mAb N418 is required for amplification of the serum antibody response. These studies demonstrate that a single-step, facilitated-delivery of small amounts of protein antigen to dendritic cells in vivo can give very rapid and high antibody responses. The approach may be particularly useful for vaccination immediately before or just after exposure to a pathogen and may enhance the utility of subunit antigens as immunogens.
Vaccines based on attenuated pathogens have been extraordinarily successful in preventing disease (1, 2). However, for some pathogens, such as HIV-1, it has become increasingly apparent that alternative approaches will be required (3, 4). Further, induction of protective humoral and cellular responses may require administration of individually optimized reagents that are combined in a vaccine. In terms of the induction of humoral responses, subunit vaccination has met with some success but also has marked limitations. In particular, the immunogenicity of subunit proteins is often poor, requiring the use of adjuvant and multiple immunization regimes.
In recent years, an exciting approach based on targeting antigen to antigen-presenting cells in vivo has been developed (5). The approach avoids the use of adjuvant and leads to robust antibody responses in a number of cases (6–11). Initially antigen was targeted to MHC class II molecules on the surface of antigen-presenting cells by coupling antigen to anti-MHC class II mAbs. Later, targeting to specific dendritic cell markers was used (11–13), and most recently the use of Fc receptor I (CD64) as a targeting molecule has been reported (14). Most studies have focused on secondary IgG responses, although some evidence for notable primary IgG responses has been provided (6, 7). It is arguable that, if the primary IgG response is rapid enough, subunit vaccines may afford a protective role if given immediately before or just after exposure to a pathogen. For this reason, we have investigated the limits of an in vivo-targeted approach in terms of the speed of the antibody response and the amount of antigen required to elicit a robust response.
The mAb chosen for targeting in this study is N418 (15), a hamster IgG that binds to the CD11c molecule on the surface of mouse dendritic cells. Unlike its human analog, CD11c is a dendritic cell-specific surface protein in mice. Although the exact cellular role of CD11c is unclear, binding of mAb N418 has been demonstrated not to interfere with the antigen presentation process of dendritic cells (15) and has been reported to induce an antibody response to itself after a single inoculation (16). Furthermore, high levels of CD11c are found on immature dendritic cells (17), which are most efficient in antigen capture, leading to the activation of the immune system (17). Thus, mAb N418 is an excellent candidate for antigen delivery. The reporter protein antigen we chose is a polyclonal goat anti-hamster IgG antibody, which binds well to mAb N418 and is a foreign protein to the mouse. Here we establish that immunization with the antigen N418 complex induces rapid primary IgG responses with minimal quantities of antigen.
Materials and Methods
Antibodies.
N418 hybridoma [American Type Culture Collection (ATCC)] was grown in the Integra CELLine CL350 with RPMI medium 1640 supplemented with FCS. Antibody was purified with protein A-Sepharose 4 Fast Flow (Amersham Pharmacia). Purified protein was quantitated at OD280 and by SDS gel staining. Goat anti-Armenian hamster IgG (with minimum cross-reaction to mouse IgG), mouse anti-goat IgG, and donkey anti-goat IgG polyclonal IgGs were purchased from Jackson ImmunoResearch. Hamster anti-trinitrophenol (TNP) IgG was purchased from PharMingen. Goat anti-biotin IgG was purchased from Pierce.
Immunization.
Immunogens containing two different antibodies for the targeted delivery and control studies were premixed and equilibrated at room temperature for a minimum of 1 h in PBS before injection. Each group contained 3–4 mice. Each mouse was given a 25-μl intradermal injection of the solution containing 0.5 μg of each antibody, unless otherwise indicated. Endotoxin levels of the antibodies were determined to be less than 1 endotoxin unit/ml per immunization (BioWhittaker). Female CBA mice (Scripps Animal Facility and Harlan–Sprague–Dawley) were 4–8 weeks old and BALB/c mice (Scripps Animal Facility) were 6–8 weeks old. Each group consisted of 3–4 mice. One mouse per group was bled 1–5 days before immunization to establish baseline antiserum. The mice were bled periodically after the immunization on the days indicated.
ELISA.
All ELISA plates were coated with goat anti-Armenian hamster IgG or anti-biotin antibody, depending on the model antigen received by the individual mouse. Antigens were coated at 2 μg/ml PBS. The backgrounds of individual sera were determined by using wells not coated with antigen. Sera were diluted in 0.02% Tween-20 and 1% BSA in PBS. The dilution factor was 1:200 unless otherwise specified. Mouse serum antibody was detected with alkaline phosphatase-conjugated goat anti-mouse IgG antibody (Jackson ImmunoResearch) and p-nitrophenyl phosphate substrate tablets (Sigma). Phosphatase reactions were stopped by addition of 0.5 M EDTA at a uniform time point for each set assayed. The hydrolysis product, p-nitrophenolate, was quantitated by absorbance at 405 nm. ELISAs of the graphs presented in each figure were performed as a complete set; to monitor consistency, commercial mouse anti-goat IgG antibody (Jackson ImmunoResearch) was used as internal standard for individual plates of each set. Serum IgG titers were determined by serial 10-fold dilutions, starting from 1:100. Titer values were assigned as the highest dilution at which the optical density was 2 SDs higher than the optical density of the baseline antiserum at equivalent dilutions. The isotype determination was performed with a Zymed mouse isotyping kit.
Results
Dendritic Cell Targeting Enhances Specific Antibody Responses.
To determine whether antibody responses can be enhanced by targeting dendritic cells with the hamster mAb N418, immunizations were performed with goat anti-hamster IgG antibody as the model antigen. The specificity and potential efficacy of this approach were evaluated by comparing separate groups of mice immunized with either a mixture of mAb N418 and goat anti-hamster IgG antibody or a control immunogen. Five groups consisting of 3–4 mice per group were immunized as follows: group 1, polyclonal goat anti-hamster IgG antibody; group 2, hamster anti-mouse CD11c, mAb N418; group 3, hamster anti-TNP antibody; group 4, mixture of goat anti-hamster antibody and mAb N418; and group 5, mixture of goat anti-hamster IgG antibody and hamster anti-TNP antibody. Group 1 served to provide the baseline mouse antibody response to the goat protein. Group 4 assayed the antibody response in mice receiving the targeted goat protein. The hamster anti-TNP antibody (group 3) is the species-matched control for N418. Groups receiving only mAb N418 (group 2) or hamster anti-TNP (group 3) were controls to verify that the mouse antibody response was caused by the presence of the goat protein instead of any cross-reactivity of mouse anti-hamster and anti-goat specificities. The mixture of goat anti-hamster IgG and hamster anti-TNP (group 5) antibodies provided a control for a nontargeted complex of goat and hamster antibodies.
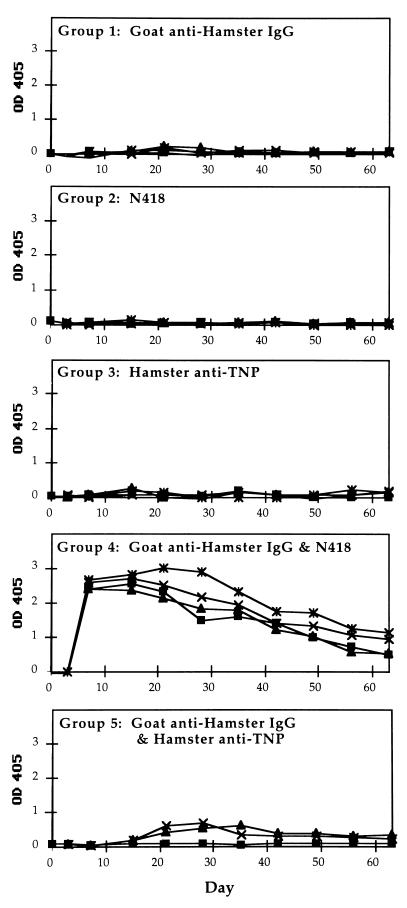
In the first experiment, we examined the time course of serum IgG levels specific for goat anti-hamster antibody in immunized CBA mice (Fig. 1). Each mouse in this study received 0.5 μg of each of the indicated proteins on day 0. The results of these experiments clearly show that the goat antibody alone did not induce the production of detectable goat-specific IgG. The same is true for the groups that received only mAb N418 or hamster anti-TNP. The mice that received the mixture of mAb N418 and goat anti-hamster antibody displayed a high IgG response on day 7. The IgG serum titers were 1:10,000 for three mice and 1:100,000 for one mouse (mean, 1:32,500). The serum IgG levels of the control groups remained undetectable at a 1:75 dilution (data not shown).
Figure 1.
Time course of IgG anti-goat response in CBA mice immunized with various antigens. All sera were diluted 300× for this ELISA assay. Four mice were immunized in each group, except for group 5, which included 3 mice. The antigen received by each group was as follows: Group 1, polyclonal goat anti-hamster IgG antibody; Group 2, mAb N418; Group 3, hamster anti-TNP antibody; Group 4, mixture of goat anti-hamster antibody and mAb N418; and Group 5, mixture of goat anti-hamster IgG antibody and hamster anti-TNP antibody. Each mouse was given 0.5 μg of each indicated protein on day 0. The data points shown for day 0 correspond to sera obtained before immunization. Postimmunization serum samples were collected on days 3 and 7, followed by weekly collections.
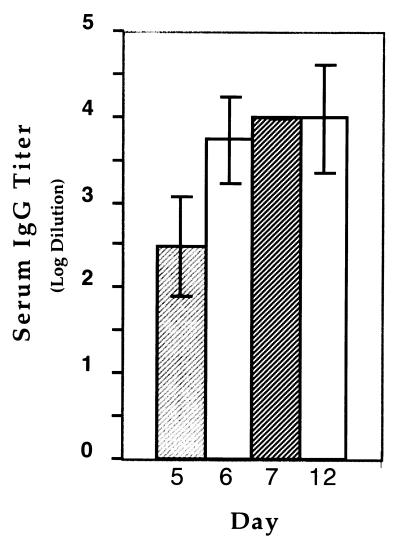
To better monitor the time course of the response to targeted immunization, CBA mice were assayed for serum IgG specific for the goat antibody, on the 5th, 6th, and 7th days after immunization (Fig. 2). Three groups of 12 mice were immunized with the N418-goat antibody mixture, hamster anti-TNP goat antibody mixture, or the goat antibody alone. Each group of 12 mice was divided into three sets of 4 mice. Set 1 was assayed for goat-specific antibody on day 5, set 2 was assayed on day 6, and set 3 was assayed on day 7. On day 12, all mice were assayed for goat antibody-specific serum IgG. Goat antibody-specific IgG serum titers for the targeted immunization were as follows: day 5 titer, 1:100–1:1,000 (mean, 1:550); day 6 titer, 1:1,000 to 1:10,000 (mean: 1:7,750); and day 7 titer: 1:10,000 for all four mice. The control groups had no detectable serum antibody on these days. In this second set of experiments, some of the mice developed specific anti-goat titers by day 12. Six out of 12 mice that received 0.5 μg of goat anti-hamster antibody had detectable goat-specific antiserum; the titer of one mouse reached 1:10,000 whereas the rest ranged from 1:100 to 1:1000 (mean titer: 1:1175; data not shown). The mice that received mixtures of hamster anti-TNP and goat anti-hamster IgG antibodies generated titers that ranged from 1:100 to 1:1,000 (mean titer: 1:775). In comparison, all 12 mice that received the targeted vaccine had titers ranging from 1:1000 to 1:100,000 (mean titer: 1:22,667). Altogether, these experiments showed that the titers generated in response to the targeted antigens were higher, occurred more rapidly, and were more consistently observed than those of the control immunizations.
Figure 2.
Goat antibody-specific serum IgG titer of CBA mice that received the targeted immunization. Antigen-specific IgG sera obtained on days 5–7 and 12 postimmunization were assayed by ELISA at serial 10× dilutions, starting at 100×. Each column represents the averaged value of sera collected on days 5–7 and 12. The values presented for days 5–7 each consisted of 4 mice; on day 7, all 4 mice had IgG titers of 1:10,000. The value for day 12 is the average of all 12 mice used in the study. The standard errors are as shown.
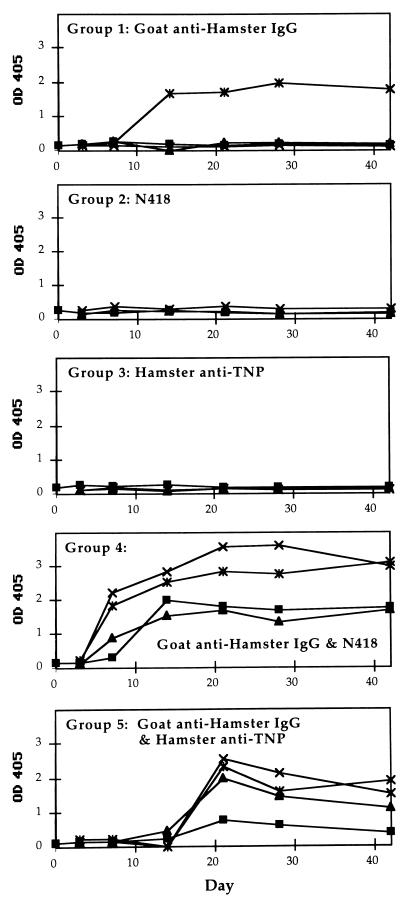
To determine whether these observations are unique to CBA mice, separate groups of BALB/c mice were immunized. A similar pattern of results was obtained when the antigens described in Fig. 1 were given to BALB/c mice (Fig. 3). Three out of 4 mice that received the targeted vaccine responded by day 7. The goat anti-hamster antibody-specific IgG titers ranged from 1:10,000 to 1:100,000 for the three mice (mean: 1:40,000). The one mouse that did not respond by day 7 was unique in our study with BALB/c mice and did have a high level of goat-specific serum antibody at the next blood draw on day 14. The mice that received only the hamster antibodies (groups 2 and 3) had no detectable serum antibody response on day 7. Although none of the BALB/c control groups generated any IgG response by day 7, these mice (groups 1–3 and 5) also exhibited considerable individual variations in response at later time points. One mouse in the group that received the goat antibody alone responded strongly by day 14, and the group that received the goat protein in combination with the hamster isotype control responded on or after day 14. Analogous to the CBA mice, only the BALB/c mice that received the N418-goat antibody complex generated antigen-specific IgG at an accelerated rate. These results showed that the targeted vaccine is effective in different strains of mice.
Figure 3.
Time course of IgG anti-goat response in BALB/c mice immunized with various antigens. Each group consisted of 3–4 mice. The antigen received by each group was as follows: Group 1, polyclonal goat anti-hamster IgG antibody; Group 2, mAb N418; Group 3, hamster anti-TNP antibody; Group 4, mixture of goat anti-hamster antibody and mAb N418; and Group 5, mixture of goat anti-hamster IgG antibody and hamster anti-TNP antibody. Each mouse received 0.5 μg of each of the indicated protein on day 0. The data points shown for day 0 correspond to sera obtained before immunization.
Targeted Immunization Produces Antibody Responses at Low Antigen Concentrations.
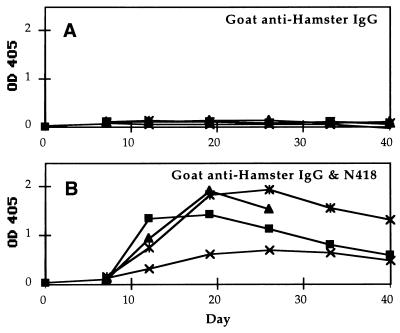
To determine whether 0.5 μg of protein antigen is the minimum threshold for triggering antibody production in mice, CBA mice were given a single immunization of 0.05 μg of goat anti-hamster antibody or the N418-goat antibody mixture at 0.05 μg each. At this dosage, antigen-specific IgG responses were not observed on day 7 in the mice that received the targeted vaccine. However, at day 12, all animals of the targeted group had goat-specific serum titers between 1:100 to 1:1000. In contrast, the mice that received goat anti-hamster antibody alone did not produce any measurable goat-specific IgG (Fig. 4). Thus, the targeted immunization enhanced antibody response even at nanogram dosage.
Figure 4.
Time course of IgG anti-goat response in CBA mice immunized with low doses of goat antibody. (A) Results in mice that received 0.05 μg of goat anti-hamster IgG antibody. (B) Results in mice that received 0.05 μg each of the goat anti-hamster IgG and mAb N418. The data points shown for day 0 correspond to sera obtained before immunization. Postimmunization serum samples were collected weekly, starting from day 7. One mouse examined in B died on day 28.
Specific Interaction Between the Goat Antibody and mAb N418 Is Required for an Enhanced Antibody Response.
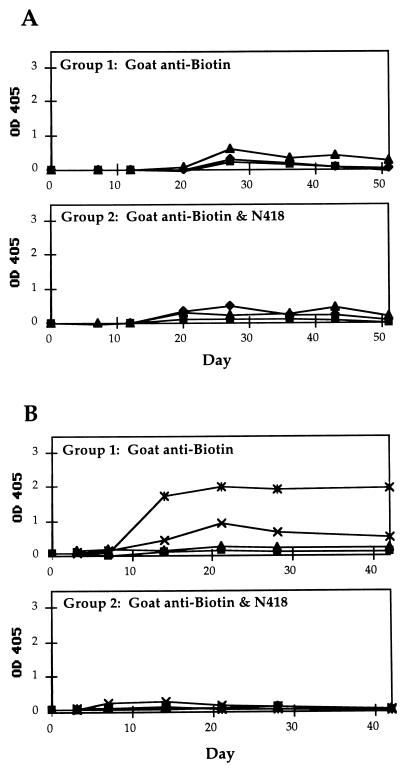
To eliminate the possibility that the amplified antibody response seen in mice was caused by nonspecific stimulation by mAb N418, mice were immunized with a mixture of mAb N418 and goat anti-biotin antibody. ELISA assays demonstrated that there is little or no interaction between the goat anti-biotin antibody and mAb N418. As shown in Fig. 5, the presence of mAb N418 did not amplify the antibody response to goat anti-biotin antibody in CBA or BALB/c mice. In fact, it seemed to have a slightly suppressive effect in BALB/c mice (Fig. 5B). Thus direct interaction between the antigen and mAb N418 is required for the amplification effect. This finding is in direct agreement with a previous study in which mice were immunized with a single dose of mAb N418 and a noninteracting rat antibody J1.2; no rat-specific antibody was detectable in the mice serum (16). In that study, a moderate antibody response against N418 (serum titer of 1:100 for 100 ng of N418) was reported by day 10 (16).
Figure 5.
Time course of IgG anti-goat responses in mice immunized with mAb N418 and a noninteracting goat antigen. (A) IgG responses of individual CBA mice. Group 1 received 0.5 μg of goat anti-biotin antibody and Group 2 received 0.5 μg each of goat anti-biotin antibody and mAb N418 on day 0. Blood was drawn periodically after immunization, starting from day 7. (B) Time course plot of BALB/c mice, data presented as described for A. Note the lack of response in the mice that received the mixture of goat anti-biotin antibody and mAb N418.
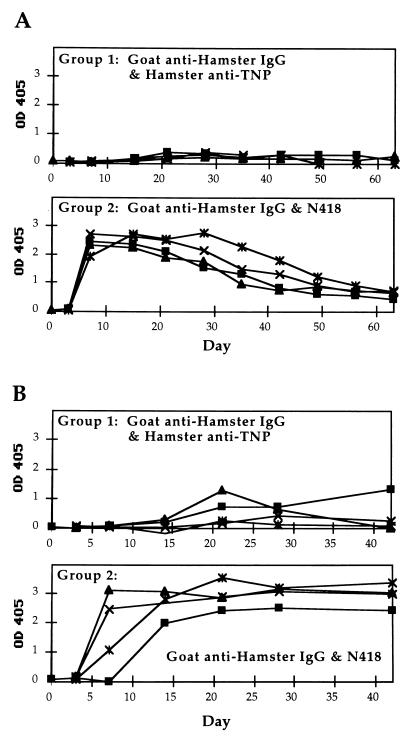
To further explore the requirement for targeting, CBA and BALB/c mice were given mAb N418 and goat anti-hamster antibody separately by injecting each protein at different sites of the same animal. Fig. 6 shows that, for both CBA and BALB/c mice, this approach resulted in vigorous antibody responses. In some studies, separate administration of mAb N418 and goat protein resulted in higher IgG titers than the premixed proteins. Separate injections of hamster anti-TNP and goat anti-hamster IgG antibodies did not result in an amplified response. Similarly, injection of mAb N418 and goat anti-biotin antibody failed to induce serum IgG production (data not shown). Potent serum antibody responses were also observed by day 7, when the goat antibody was injected at a different site one day after the injection of mAb N418, but the effect was not as strong as the simultaneous injection at separate sites (data not shown).
Figure 6.
Time course of IgG anti-goat responses in mice that received separate injections of hamster and goat antibodies. Each group consisted of 4 mice. (A) IgG response of individual CBA mice. Group 1 was given 0.5 μg each of goat anti-hamster IgG and hamster anti-TNP antibodies. Group 2 was given 0.5 μg each of goat anti-hamster IgG antibody and mAb N418. The data points shown for day 0 correspond to sera obtained before immunization. Postimmunization serum samples were collected on days 3 and 7, followed by weekly collections. (B) IgG response of individual BALB/c mice. Data presented as described for A.
Targeted Immunization Produced Antibody Responses Predominately of the IgG1 Isotype.
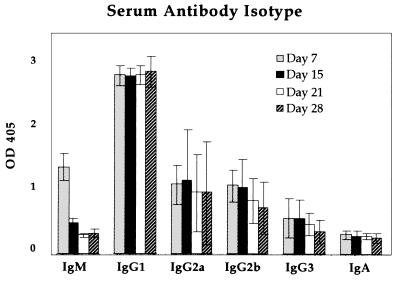
Antibody isotypes have been correlated with different mechanisms of the immune response (18). The isotype of CBA serum antibody specific for the goat antigen was determined on days 7, 15, 21, and 28 postimmunization of the targeted immunogen (Fig. 7). Significant levels of IgG1, IgG2a, and IgG2b were present in the antisera, although the serum levels of the latter two subclasses showed considerable variation in different individual animals. IgG1 was the predominate isotype, in agreement with previous findings for adjuvant-independent (13) as well as -dependent (19) protein antigens. A similar isotype profile was observed for CBA mice that received separate injections of mAb N418 and goat antibody; there was a slightly higher level of IgG2b, but the difference fell within the error range. The BALB/c mice that received the mixture of mAb N418 and goat antibody had the same isotype distribution as shown in Fig. 7. IgG1 also predominated in the BALB/c mice that were able to produce measurable serum titers after receiving only the goat protein without mAb N418 (data not shown).
Figure 7.
Isotype of goat antibody-specific antisera of CBA mice that received the targeted immunization. Breakdown of sera isotype was shown for samples obtained on days 7, 15, 21, and 28 postimmunization. Each column represents the averaged value of 4 mice. The standard errors are as shown.
Discussion
The findings presented here demonstrate that high levels of antigen-specific IgG can be generated within days of a single-step immunization by targeting antigen to dendritic cells. Our method used submicrogram quantities of antigen to induce serum titers up to 1:100,000 within 1 week. This approach of targeting antigen to dendritic cells may have multiple applications in promoting rapid immune responses in general.
In the last decade, there has been great interest in using dendritic cells to boost the immune response. Most of these studies used whole-cell vaccinations, that involved the isolation of dendritic cells from the vaccine recipient or isogenic animal, pulsing them with peptides (20), cell lysates (21, 22), or RNA (23), followed by injection into the animal or human host. These methods have resulted in dramatic protection or recovery from tumors (21–23) or viral infection (20) in the recipients. Despite the success in these studies, cell-free vaccines are highly desirable; dendritic cells are difficult and expensive to culture, and host-specific whole-cell vaccines are not easily available. Preliminary work with dendritic cell exosomes (24) showed great anti-tumor potential, but this still requires host-specific cell culturing.
As described in the introduction, a promising method of directed antigen presentation is in vivo immunotargeting (5), in which antibodies to antigen-presenting cell surface molecules are chemically conjugated to an antigen and injected into mice. Most immunotargeting studies have used general antigen-presenting cell surface molecules, such as Fc receptors (14, 25, 26), MHC (6–9, 13, 25–27), IgG (28), and IgD (29). A few studies have used antibodies specific to dendritic cells and have reported increases in antigen-specific serum antibody titers relative to immunization with nontargeted antigen (12, 13). These studies used significantly greater quantities of antibody (5–25 μg) than used here (0.05–0.5 μg) and often entailed multiinjection regimes; the kinetics of the primary humoral response were not investigated. In this study we have shown that relatively small amounts of antigen administered as a single dose can elicit potent IgG responses in a very short time after immunization, using a dendritic cell-targeted approach. Furthermore, the response is elicited more reliably than that elicited by using nontargeted immunization.
Clearly, many more studies are required to determine whether the approach discussed here would be successful in enhancing human antibody responses in a vaccine setting. Nevertheless, the principle of rapid effective single-step immunization is established and can be seen to have considerable potential in a number of vaccine scenarios. For instance, vaccinations could be considered for persons required to enter, at short notice, an area of known risk of exposure to a pathogen, as during an outbreak or in a military situation. The possibility of successful postexposure prophylaxis would also be enhanced, given a rapid induction of antibody responses. For example, a vaccine approach capable of inducing protective antibody response with a single-dose would be highly valuable, in combination with antibiotics, in reducing mortality from a covert anthrax attack. The current anthrax vaccine is effective but requires a multiple (six)-dose regime (30). In outbreaks of Argentine hemorrhagic fever, which is caused by Junin virus, death can be prevented if high antibody titers are present within 8 days of onset of symptoms (31). Antibody is normally provided by passive immunization, but more convenient active immunization could be considered for a rapid antibody response. Finally, the ability to stimulate effective antibody responses with small amounts of protein could be of considerable importance in strategies that use subunit proteins as components of vaccines or may even aid in the delivery and efficacy of DNA vaccines.
Acknowledgments
We are grateful to Drs. Charles Surh and Lindsay Whitton for their insightful discussions. We thank Drs. Paul Parren and Pascal Poignard for discussions on the manuscript and Drs. Juan Carlos Gonzalez, Dong-sup Lee, Munir Alam, and Stella Redpath for their assistance. Danielle Foster, Christina Lin, Fatima Garcia del Rey, and Ann Hessell have provided valuable technical assistance for this work. This work was supported by Defense Advanced Research Projects Agency Grant MDA 972-97-1 (to P.G.), National Institutes of Health Grants AI 33292 and AI 39808 (to D.R.B.), and National Institute of Allergy and Infectious Diseases immunology training Grant T32 AI07266-16 (to H.W.). P.G. is a Scholar of the Leukemia Society of America.
References
- 1.Hilleman M R. Nat Med. 1998;4:507–514. doi: 10.1038/nm0598supp-507. [DOI] [PubMed] [Google Scholar]
- 2.Ellis R W. In: Vaccines. Plotkin S A, Orenstein W A, editors. Philadelphia: Saunders; 1999. pp. 881–901. [Google Scholar]
- 3.Letvin N L. Science. 1998;280:1875–1880. doi: 10.1126/science.280.5371.1875. [DOI] [PubMed] [Google Scholar]
- 4.Burton D R, Moore J P. Nat Med. 1998;4:495–498. doi: 10.1038/nm0598supp-495. [DOI] [PubMed] [Google Scholar]
- 5.Barber B H. Semin Immunol. 1997;9:293–301. doi: 10.1006/smim.1997.0085. [DOI] [PubMed] [Google Scholar]
- 6.Berg S F, Mjaaland S, Fossum S. Eur J Immunol. 1994;24:1262–1268. doi: 10.1002/eji.1830240604. [DOI] [PubMed] [Google Scholar]
- 7.Carayanniotis G, Barber B H. Vaccine. 1990;8:137–144. doi: 10.1016/0264-410x(90)90136-a. [DOI] [PubMed] [Google Scholar]
- 8.Snider D P. J Immunol. 1992;148:1163–1170. [PubMed] [Google Scholar]
- 9.Carayanniotis G, Barber B H. Nature (London) 1987;327:59–61. doi: 10.1038/327059a0. [DOI] [PubMed] [Google Scholar]
- 10.Lees A, Morris S C, Thyphronitis G, Holmes J M, Inman J K, Finkelman F D. J Immunol. 1990;145:3594–3600. [PubMed] [Google Scholar]
- 11.Carayanniotis G, Skea D L, Luscher M A, Barber B H. Mol Immunol. 1991;28:261–267. doi: 10.1016/0161-5890(91)90072-r. [DOI] [PubMed] [Google Scholar]
- 12.Sornasse T, Flamand V, De Becker G, Thielemans K, Urbain J, Leo O, Moser M. In: Dendritic Cells in Fundamental and Clinical Immunology, Advances in Experimental Medicine and Biology. Kamperdijk E W A, Nieuwenhuis P, editors. Vol. 329. New York: Plenum; 1993. pp. 299–303. [Google Scholar]
- 13.Skea D L, Barber B H. J Immunol. 1993;151:3557–3568. [PubMed] [Google Scholar]
- 14.Guyre P M, Graziano R F, Goldstein J, Morganelli P M, Wardwell K, Howell A L. Cancer Immunol Immunother. 1997;45:146–148. doi: 10.1007/s002620050418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Metlay J P, Witmer-Pack M D, Agger R, Crowley M T, Lawless D, Steinman R M. J Exp Med. 1990;171:1753–1771. doi: 10.1084/jem.171.5.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Finkelman F D, Lees A, Birnbaum R, Gause W C, Morris S C. J Immunol. 1996;157:1406–1414. [PubMed] [Google Scholar]
- 17.Steinman R M, Pack M, Inaba K. Immunol Rev. 1997;156:25–37. doi: 10.1111/j.1600-065x.1997.tb00956.x. [DOI] [PubMed] [Google Scholar]
- 18.Snapper C M, Mond J J. Immunol Today. 1993;14:15–17. doi: 10.1016/0167-5699(93)90318-F. [DOI] [PubMed] [Google Scholar]
- 19.Gupta R K, Relyveld E H, Lindblad E B, Bizzini B, Ben-Efraim S, Goptta C K. Vaccine. 1993;11:293–306. doi: 10.1016/0264-410x(93)90190-9. [DOI] [PubMed] [Google Scholar]
- 20.Shimizu Y, Guidotti L G, Fowler P, Chisari F V. J Immunol. 1998;161:4520–4529. [PubMed] [Google Scholar]
- 21.Fields R C, Shimizu K, Mule J J. Proc Natl Acad Sci USA. 1998;95:9482–9487. doi: 10.1073/pnas.95.16.9482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Holtl L, Rieser C, Papesh C, Ramoner R, Bartsch G, Thurnher M. Lancet. 1998;352:1358–1361. doi: 10.1016/s0140-6736(05)60748-9. [DOI] [PubMed] [Google Scholar]
- 23.Smita K N, Boczkowski D, Morse M, Cumming R I, Lyerly H K, Gilboa E. Nat Biotech. 1998;16:364–369. doi: 10.1038/nbt0498-364. [DOI] [PubMed] [Google Scholar]
- 24.Zitvogel L, Regnault A, Lozier A, Wolfers J, Flament C, Tenza D, Ricciardi-Castagnoli P, Raposo G, Amigorena S. Nat Med. 1998;4:594–600. doi: 10.1038/nm0598-594. [DOI] [PubMed] [Google Scholar]
- 25.Snider D P, Segal D M. J Immunol. 1987;139:1609–1616. [PubMed] [Google Scholar]
- 26.Snider D P. J Immunol. 1992;148:1163–1170. [PubMed] [Google Scholar]
- 27.Snider D P, Kaubisch A, Segal D M. J Exp Med. 1990;171:1957–1963. doi: 10.1084/jem.171.6.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kawamura H, Berzofsky J A. J Immunol. 1986;136:58–65. [PubMed] [Google Scholar]
- 29.Lees A, Morris S C, Thyphronitis G, Holmes J M, Inman J K, Finkelman F D. J Immunol. 1990;145:3594–3600. [PubMed] [Google Scholar]
- 30.Russell P K. Emerging Infect Dis. 1999;5:531–533. doi: 10.3201/eid0504.990413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Enria D A, Fernandex N J, Briggiler A M, Levis S C, Maiztegiu J I. Lancet. 1984;4:255–256. doi: 10.1016/s0140-6736(84)90299-x. [DOI] [PubMed] [Google Scholar]