Abstract
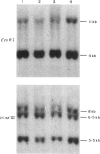
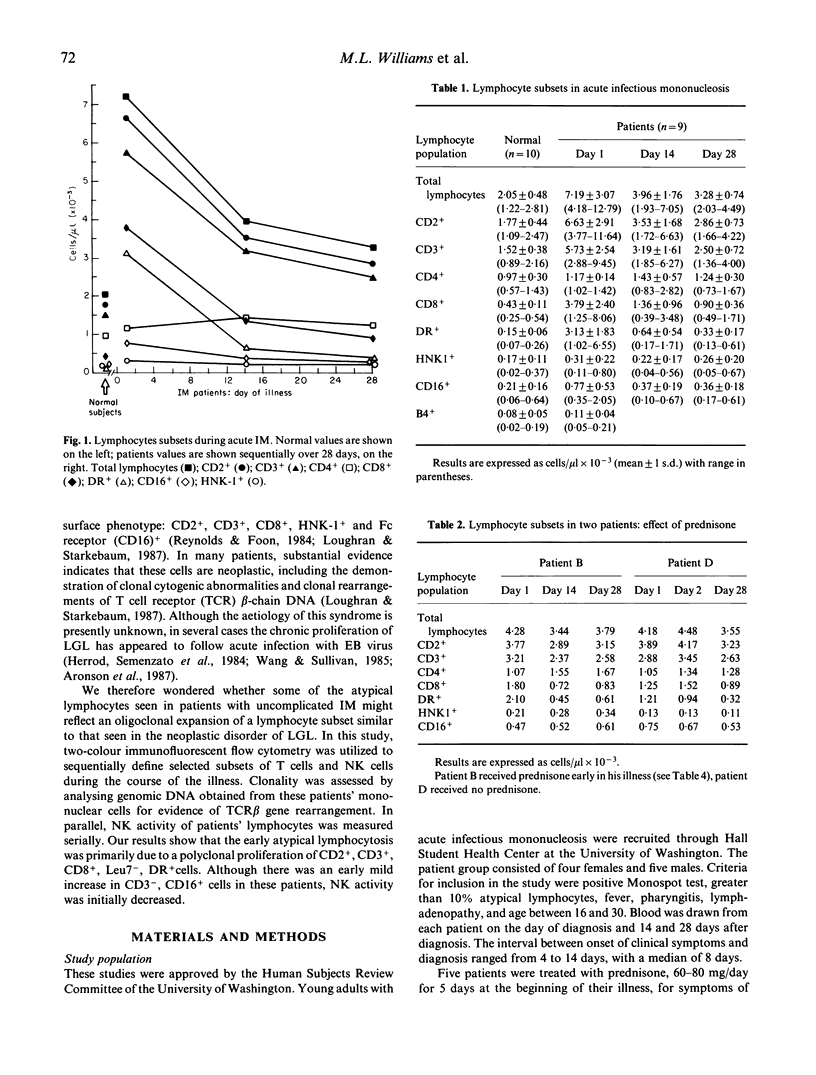
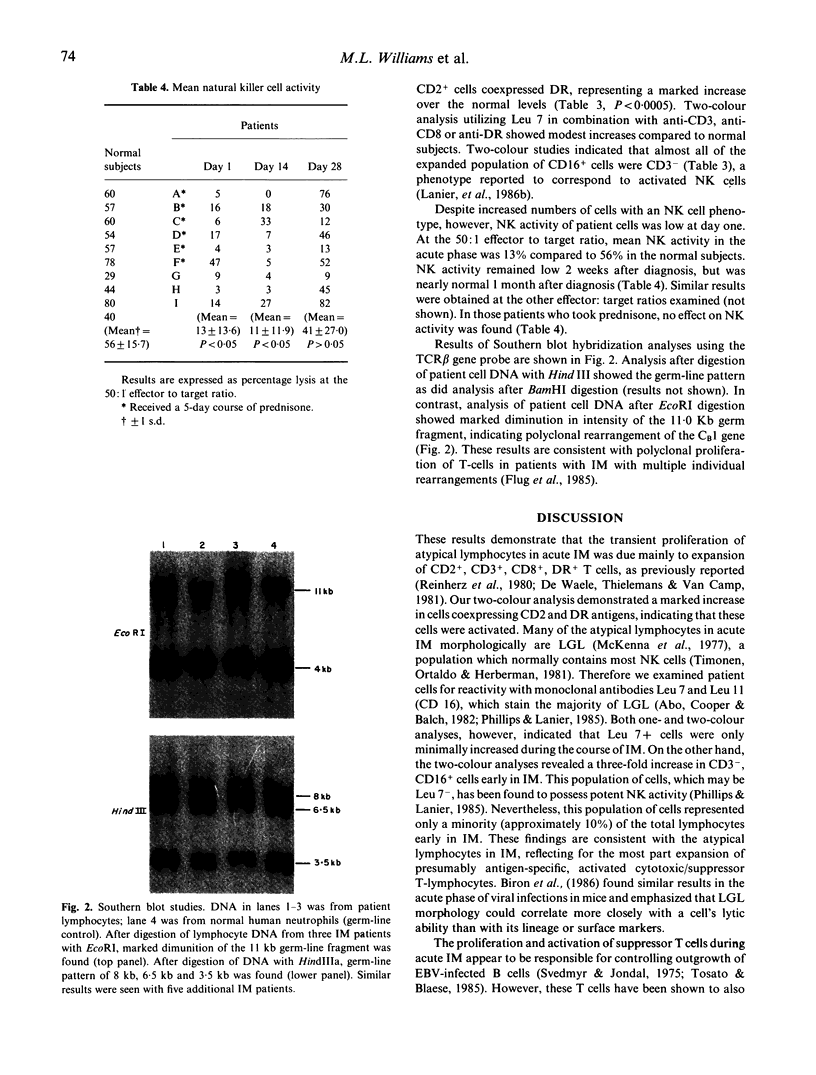
In acute infectious mononucleosis large numbers of atypical lymphocytes proliferate in response to B cells infected with Epstein-Barr virus, generally resulting in a self-limited illness. Although both T-cells and NK cells are known to be involved, the precise origin of the large granular lymphocytes in this disorder is incompletely understood. Using two-colour immunofluorescent flow cytometry, we sequentially examined the phenotype of selected T cell and NK cell subsets from nine patients with infectious mononucleosis. In parallel, we determined whether these lymphocytes utilized a restricted repertoire of the T cell receptor gene and also measured their NK activity. Our results show that in acute infectious mononucleosis there was a greater than three-fold increase in T lymphocytes with the phenotype CD2+, CD3+, CD8+ and DR+. A modest increase in Leu7(HNK1)+ and CD4+ T cells was also seen. In addition, there was a three-fold increase in cells coexpressing CD3- and CD16+, the phenotype reported to represent most NK cells. In spite of this latter finding, however, a marked decrease in NK function was found at the time of diagnosis, gradually returning to normal by day 28. Finally, Southern blot analysis of DNA from patient lymphocytes showed polyclonal rearrangements of the T cell receptor beta chain gene. These studies indicate that the proliferation of activated suppressor/cytotoxic T lymphocytes in acute infectious mononucleosis is polyclonal and is associated with transient depression of NK function.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abo T., Cooper M. D., Balch C. M. Characterization of HNK-1+ (Leu-7) human lymphocytes. I. Two distinct phenotypes of human NK cells with different cytotoxic capability. J Immunol. 1982 Oct;129(4):1752–1757. [PubMed] [Google Scholar]
- Aronson F. R., Dempsey R. A., Allegretta M., André-Schwartz J., Poldre P. A., Hillyer C. D., Parkinson D. R., Rudders R. A., Schwartz R. S., Mier J. W. Malignant granular lymphoproliferation after Epstein-Barr virus infection: partial immunologic reconstitution with interleukin-2. Am J Hematol. 1987 Aug;25(4):427–439. doi: 10.1002/ajh.2830250409. [DOI] [PubMed] [Google Scholar]
- Beall S. S., Lawrence J. V., Bradley D. A., Mattson D. H., Singer D. S., Biddison W. E. Beta-chain gene rearrangements and V beta gene usage in DPw2-specific T cells. J Immunol. 1987 Aug 15;139(4):1320–1325. [PubMed] [Google Scholar]
- Biron C. A., Natuk R. J., Welsh R. M. Generation of large granular T lymphocytes in vivo during viral infection. J Immunol. 1986 Mar 15;136(6):2280–2286. [PubMed] [Google Scholar]
- Brewster F. E., Byron K. S., Sullivan J. L. Immunoregulation during acute infection with Epstein-Barr virus: dynamics of interferon and 2',5'-oligoadenylate synthetase activity. J Infect Dis. 1985 Jun;151(6):1109–1115. doi: 10.1093/infdis/151.6.1109. [DOI] [PubMed] [Google Scholar]
- Cheeseman S. H. Infectious mononucleosis. Semin Hematol. 1988 Jul;25(3):261–268. [PubMed] [Google Scholar]
- De Waele M., Thielemans C., Van Camp B. K. Characterization of immunoregulatory T cells in EBV-induced infectious mononucleosis by monoclonal antibodies. N Engl J Med. 1981 Feb 19;304(8):460–462. doi: 10.1056/NEJM198102193040804. [DOI] [PubMed] [Google Scholar]
- Flug F., Pelicci P. G., Bonetti F., Knowles D. M., 2nd, Dalla-Favera R. T-cell receptor gene rearrangements as markers of lineage and clonality in T-cell neoplasms. Proc Natl Acad Sci U S A. 1985 May;82(10):3460–3464. doi: 10.1073/pnas.82.10.3460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes B. F., Schooley R. T., Grouse J. E., Payling-Wright C. R., Dolin R., Fauci A. S. Characterization of thymus-derived lymphocyte subsets in acute Epstein-Barr virus-induced infectious mononucleosis. J Immunol. 1979 Feb;122(2):699–702. [PubMed] [Google Scholar]
- Henle G., Henle W., Diehl V. Relation of Burkitt's tumor-associated herpes-ytpe virus to infectious mononucleosis. Proc Natl Acad Sci U S A. 1968 Jan;59(1):94–101. doi: 10.1073/pnas.59.1.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrod H. G., Wang W. C., Sullivan J. L. Chronic T-cell lymphocytosis with neutropenia. Its association with Epstein-Barr virus infection. Am J Dis Child. 1985 Apr;139(4):405–407. doi: 10.1001/archpedi.1985.02140060087037. [DOI] [PubMed] [Google Scholar]
- Hochgeschwender U., Weltzien H. U., Eichmann K., Wallace R. B., Epplen J. T. Preferential expression of a defined T-cell receptor beta-chain gene in hapten-specific cytotoxic T-cell clones. Nature. 1986 Jul 24;322(6077):376–378. doi: 10.1038/322376a0. [DOI] [PubMed] [Google Scholar]
- Junker A. K., Ochs H. D., Clark E. A., Puterman M. L., Wedgwood R. J. Transient immune deficiency in patients with acute Epstein-Barr virus infection. Clin Immunol Immunopathol. 1986 Sep;40(3):436–446. doi: 10.1016/0090-1229(86)90188-1. [DOI] [PubMed] [Google Scholar]
- Kaplan J., Shope T. C. Natural killer cells inhibit outgrowth of autologous Epstein-Barr virus-infected B lymphocytes. Nat Immun Cell Growth Regul. 1985;4(1):40–47. [PubMed] [Google Scholar]
- Kronenberg M., Siu G., Hood L. E., Shastri N. The molecular genetics of the T-cell antigen receptor and T-cell antigen recognition. Annu Rev Immunol. 1986;4:529–591. doi: 10.1146/annurev.iy.04.040186.002525. [DOI] [PubMed] [Google Scholar]
- Lanier L. L., Le A. M., Cwirla S., Federspiel N., Phillips J. H. Antigenic, functional, and molecular genetic studies of human natural killer cells and cytotoxic T lymphocytes not restricted by the major histocompatibility complex. Fed Proc. 1986 Nov;45(12):2823–2828. [PubMed] [Google Scholar]
- Loughran T. P., Jr, Starkebaum G. Large granular lymphocyte leukemia. Report of 38 cases and review of the literature. Medicine (Baltimore) 1987 Sep;66(5):397–405. [PubMed] [Google Scholar]
- Mangi R. J., Niederman J. C., Kelleher J. E., Jr, Dwyer J. M., Evans A. S., Kantor F. S. Depression of cell-mediated immunity during acute infectious mononucleosis. N Engl J Med. 1974 Nov 28;291(22):1149–1153. doi: 10.1056/NEJM197411282912202. [DOI] [PubMed] [Google Scholar]
- McKenna R. W., Parkin J., Gajl-Peczalska K. J., Kersey J. H., Brunning R. D. Ultrastructural, cytochemical, and membrane surface marker characteristics of the atypical lymphocytes in infectious mononucleosis. Blood. 1977 Sep;50(3):505–515. [PubMed] [Google Scholar]
- Pattengale P. K., Smith R. W., Perlin E. Atypical lymphocytes in acute infectious mononucleosis. Identification by multiple T and B lymphocyte markers. N Engl J Med. 1974 Nov 28;291(22):1145–1148. doi: 10.1056/NEJM197411282912201. [DOI] [PubMed] [Google Scholar]
- Phillips J. H., Lanier L. L. A model for the differentiation of human natural killer cells. Studies on the in vitro activation of Leu-11+ granular lymphocytes with a natural killer-sensitive tumor cell, K562. J Exp Med. 1985 Jun 1;161(6):1464–1482. doi: 10.1084/jem.161.6.1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinherz E. L., O'Brien C., Rosenthal P., Schlossman S. F. The cellular basis for viral-induced immunodeficiency: analysis by monoclonal antibodies. J Immunol. 1980 Sep;125(3):1269–1274. [PubMed] [Google Scholar]
- Reynolds C. W., Foon K. A. T gamma-lymphoproliferative disease and related disorders in humans and experimental animals: a review of the clinical, cellular, and functional characteristics. Blood. 1984 Dec;64(6):1146–1158. [PubMed] [Google Scholar]
- Royston I., Sullivan J. L., Periman P. O., Perlin E. Cell-mediated immunity to Epstein-Barr-virus-transformed lymphoblastoid cells in acute infectious mononucleosis. N Engl J Med. 1975 Dec 4;293(23):1159–1163. doi: 10.1056/NEJM197512042932301. [DOI] [PubMed] [Google Scholar]
- Seeley J., Svedmyr E., Weiland O., Klein G., Moller E., Eriksson E., Andersson K., van der Waal L. Epstein Barr virus selective T cells in infectious mononucleosis are not restricted to HLA-A and B antigens. J Immunol. 1981 Jul;127(1):293–300. [PubMed] [Google Scholar]
- Semenzato G., Pizzolo G., Ranucci A., Agostini C., Chilosi M., Quinti I., De Sanctis G., Vercelli B., Pandolfi F. Abnormal expansions of polyclonal large to small size granular lymphocytes: reactive or neoplastic process? Blood. 1984 Jun;63(6):1271–1277. [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Sullivan J. L., Byron K. S., Brewster F. E., Purtilo D. T. Deficient natural killer cell activity in x-linked lymphoproliferative syndrome. Science. 1980 Oct 31;210(4469):543–545. doi: 10.1126/science.6158759. [DOI] [PubMed] [Google Scholar]
- Svedmyr E., Ernberg I., Seeley J., Weiland O., Masucci G., Tsukuda K., Szigeti R., Masucci M. G., Blomogren H., Berthold W. Virologic, immunologic, and clinical observations on a patient during the incubation, acute, and convalescent phases of infectious mononucleosis. Clin Immunol Immunopathol. 1984 Mar;30(3):437–450. doi: 10.1016/0090-1229(84)90029-1. [DOI] [PubMed] [Google Scholar]
- Svedmyr E., Jondal M. Cytotoxic effector cells specific for B Cell lines transformed by Epstein-Barr virus are present in patients with infectious mononucleosis. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1622–1626. doi: 10.1073/pnas.72.4.1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarkkanen J., Saksela E., von Willebrand E., Lehtonen E. Suppressor cells of the human NK activity: characterization of the cells and mechanism of action. Cell Immunol. 1983 Jul 15;79(2):265–278. doi: 10.1016/0008-8749(83)90069-2. [DOI] [PubMed] [Google Scholar]
- Thorley-Lawson D. A., Israelsohn E. S. Generation of specific cytotoxic T cells with a fragment of the Epstein-Barr virus-encoded p63/latent membrane protein. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5384–5388. doi: 10.1073/pnas.84.15.5384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosato G., Blaese R. M. Epstein-Barr virus infection and immunoregulation in man. Adv Immunol. 1985;37:99–149. doi: 10.1016/s0065-2776(08)60339-9. [DOI] [PubMed] [Google Scholar]
- Tosato G., Magrath I., Koski I., Dooley N., Blaese M. Activation of suppressor T cells during Epstein-Barr-virus-induced infectious mononucleosis. N Engl J Med. 1979 Nov 22;301(21):1133–1137. doi: 10.1056/NEJM197911223012101. [DOI] [PubMed] [Google Scholar]
- Toyonaga B., Yoshikai Y., Vadasz V., Chin B., Mak T. W. Organization and sequences of the diversity, joining, and constant region genes of the human T-cell receptor beta chain. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8624–8628. doi: 10.1073/pnas.82.24.8624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldmann T. A., Davis M. M., Bongiovanni K. F., Korsmeyer S. J. Rearrangements of genes for the antigen receptor on T cells as markers of lineage and clonality in human lymphoid neoplasms. N Engl J Med. 1985 Sep 26;313(13):776–783. doi: 10.1056/NEJM198509263131303. [DOI] [PubMed] [Google Scholar]
- Yoshikai Y., Anatoniou D., Clark S. P., Yanagi Y., Sangster R., Van den Elsen P., Terhorst C., Mak T. W. Sequence and expression of transcripts of the human T-cell receptor beta-chain genes. Nature. 1984 Dec 6;312(5994):521–524. doi: 10.1038/312521a0. [DOI] [PubMed] [Google Scholar]
- Zinkernagel R. M., Doherty P. C. MHC-restricted cytotoxic T cells: studies on the biological role of polymorphic major transplantation antigens determining T-cell restriction-specificity, function, and responsiveness. Adv Immunol. 1979;27:51–177. doi: 10.1016/s0065-2776(08)60262-x. [DOI] [PubMed] [Google Scholar]