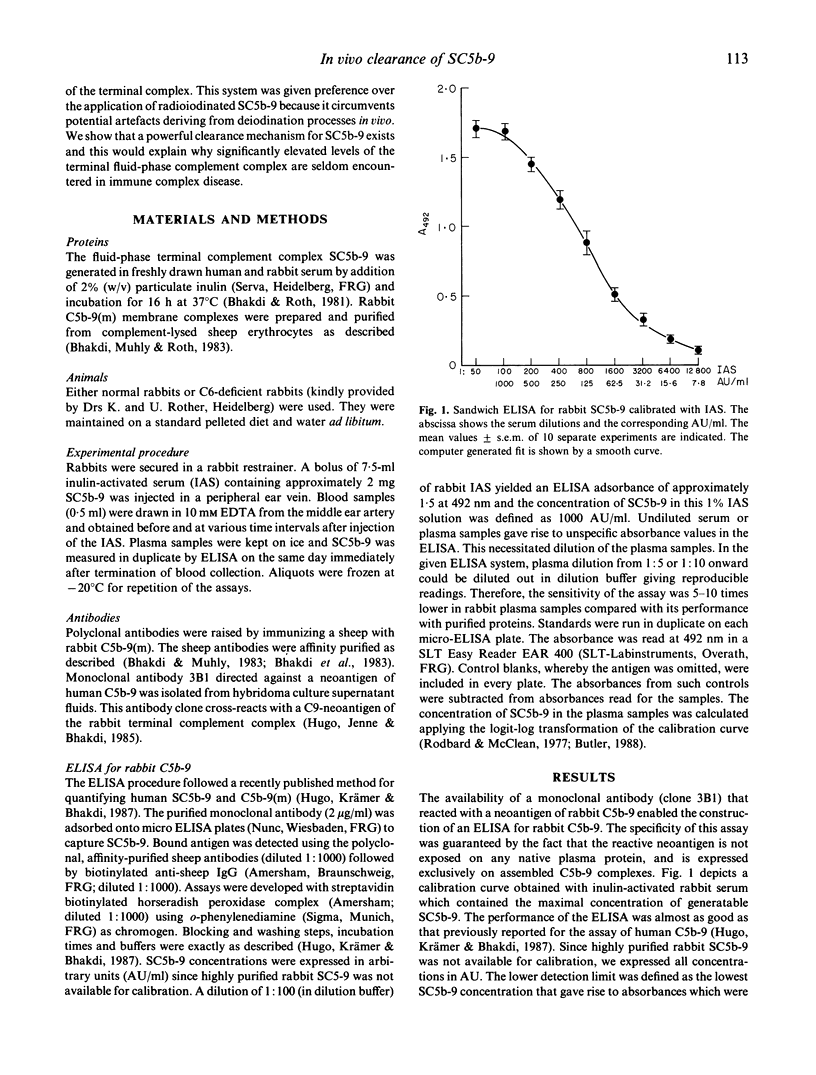
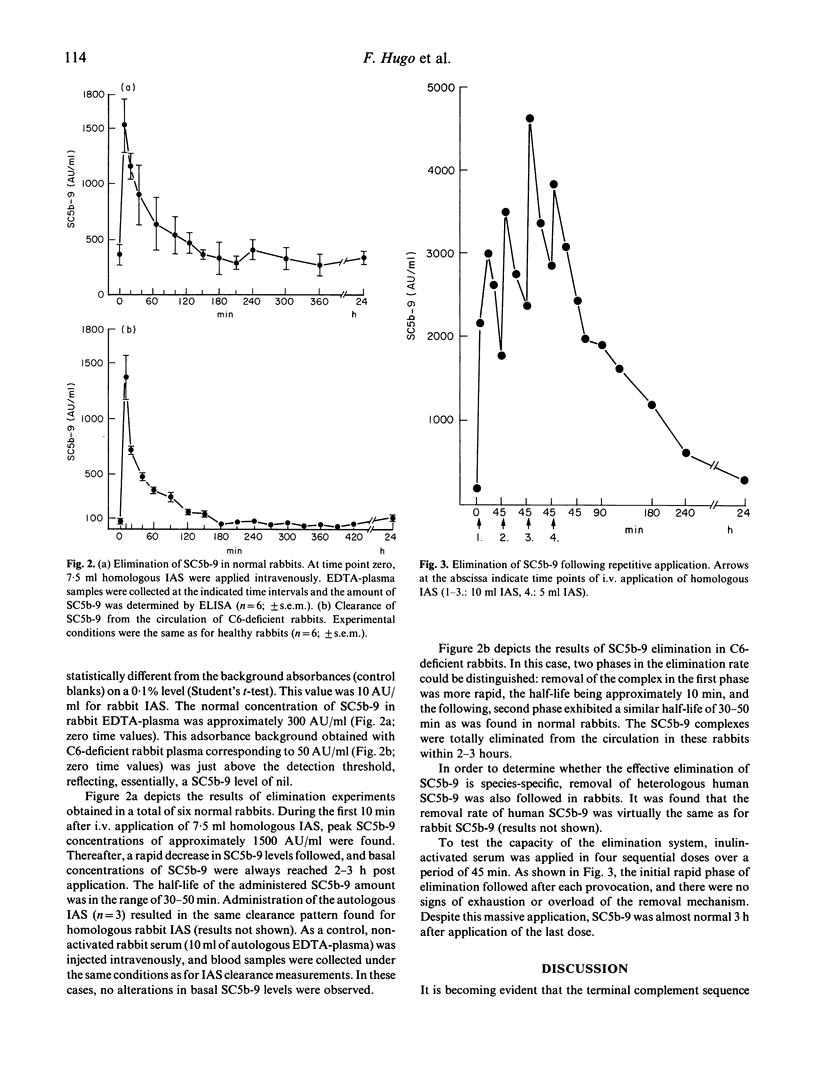
Abstract
The present study was directed at obtaining information on the in vivo elimination rate of SC5b-9, the terminal fluid-phase product of complement activation. A sandwich ELISA based on the use of mono- and polyclonal antibodies was constructed that permitted quantitation of rabbit SC5b-9 in plasma. Rabbit serum was activated with inulin in vitro to generate SC5b-9, and the activated serum was applied intravenously in normal and C6-deficient rabbits. Elimination of SC5b-9 in normal rabbits was rapid, half-life in the range of 30-50 min. No differences were noted between the clearance of homologous rabbit and heterologous human SC5b-9, SC5b-9 concentrations returned to basal levels 2-3 h after application. Plasma of C6-deficient rabbits contained no SC5b-9 and these animals displayed an even more effective clearance capacity for the complex. Quantitative considerations indicated that basal plasma SC5b-9 levels in healthy animals result from a spontaneous turnover rate of approximately 0.2% of C5-C9 components per h. When multiple doses of SC5b-9 were injected in sequence, the same half-life and total elimination time were found as with single-dose experiments. The results demonstrate the existence of an effective clearance mechanism for SC5b-9, consistent with recent findings that SC5b-9 plasma levels are very low not only in healthy adults, but also in the majority of patients with complement-consuming diseases.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alper C. A., Rosen F. S. Alper CA, Rosen FS: Studies of the in vivo behavior of human C'3 in normal subjects and patients. J Clin Invest. 1967 Dec;46(12):2021–2034. doi: 10.1172/JCI105691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhakdi S., Fassbender W., Hugo F., Carreno M. P., Berstecher C., Malasit P., Kazatchkine M. D. Relative inefficiency of terminal complement activation. J Immunol. 1988 Nov 1;141(9):3117–3122. [PubMed] [Google Scholar]
- Bhakdi S., Muhly M. A simple immunoradiometric assay for the terminal SC5b-9 complex of human complement. J Immunol Methods. 1983 Feb 25;57(1-3):283–289. doi: 10.1016/0022-1759(83)90088-1. [DOI] [PubMed] [Google Scholar]
- Bhakdi S., Muhly M., Roth M. Preparation and isolation of specific antibodies to complement components. Methods Enzymol. 1983;93:409–420. doi: 10.1016/s0076-6879(83)93054-9. [DOI] [PubMed] [Google Scholar]
- Bhakdi S., Roth M. Fluid-phase SC5b-8 complex of human complement: generation and isolation from serum. J Immunol. 1981 Aug;127(2):576–580. [PubMed] [Google Scholar]
- Bhakdi S., Tranum-Jensen J. Damage to mammalian cells by proteins that form transmembrane pores. Rev Physiol Biochem Pharmacol. 1987;107:147–223. doi: 10.1007/BFb0027646. [DOI] [PubMed] [Google Scholar]
- Biesecker G. Membrane attack complex of complement as a pathologic mediator. Lab Invest. 1983 Sep;49(3):237–249. [PubMed] [Google Scholar]
- Falk R. J., Dalmasso A. P., Kim Y., Lam S., Michael A. Radioimmunoassay of the attack complex of complement in serum from patients with systemic lupus erythematosus. N Engl J Med. 1985 Jun 20;312(25):1594–1599. doi: 10.1056/NEJM198506203122502. [DOI] [PubMed] [Google Scholar]
- Fosse E., Mollnes T. E., Ingvaldsen B. Complement activation during major operations with or without cardiopulmonary bypass. J Thorac Cardiovasc Surg. 1987 Jun;93(6):860–866. [PubMed] [Google Scholar]
- Groggel G. C., Adler S., Rennke H. G., Couser W. G., Salant D. J. Role of the terminal complement pathway in experimental membranous nephropathy in the rabbit. J Clin Invest. 1983 Dec;72(6):1948–1957. doi: 10.1172/JCI111159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horigome I., Seino J., Sudo K., Kinoshita Y., Saito T., Yoshinaga K. Terminal complement complex in plasma from patients with systemic lupus erythematosus and other glomerular diseases. Clin Exp Immunol. 1987 Nov;70(2):417–424. [PMC free article] [PubMed] [Google Scholar]
- Hugo F., Jenne D., Bhakdi S. Monoclonal antibodies against neoantigens of the terminal C5b-9 complex of human complement. Biosci Rep. 1985 Aug;5(8):649–658. doi: 10.1007/BF01116996. [DOI] [PubMed] [Google Scholar]
- Hugo F., Krämer S., Bhakdi S. Sensitive ELISA for quantitating the terminal membrane C5b-9 and fluid-phase SC5b-9 complex of human complement. J Immunol Methods. 1987 May 20;99(2):243–251. doi: 10.1016/0022-1759(87)90134-7. [DOI] [PubMed] [Google Scholar]
- Kolb W. P., Müller-Eberhard H. J. Neoantigens of the membrane attack complex of human complement. Proc Natl Acad Sci U S A. 1975 May;72(5):1687–1689. doi: 10.1073/pnas.72.5.1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koski C. L., Sanders M. E., Swoveland P. T., Lawley T. J., Shin M. L., Frank M. M., Joiner K. A. Activation of terminal components of complement in patients with Guillain-Barré syndrome and other demyelinating neuropathies. J Clin Invest. 1987 Nov;80(5):1492–1497. doi: 10.1172/JCI113231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mollnes T. E., Frøland S. S., Harboe M. Increased plasma levels of the terminal complement complex in patients with evidence of complement activation. Complement. 1985;2(2-3):175–184. doi: 10.1159/000467858. [DOI] [PubMed] [Google Scholar]
- Mollnes T. E., Lea T., Frøland S. S., Harboe M. Quantification of the terminal complement complex in human plasma by an enzyme-linked immunosorbent assay based on monoclonal antibodies against a neoantigen of the complex. Scand J Immunol. 1985 Aug;22(2):197–202. doi: 10.1111/j.1365-3083.1985.tb01871.x. [DOI] [PubMed] [Google Scholar]
- Mollnes T. E., Lea T., Mellbye O. J., Pahle J., Grand O., Harboe M. Complement activation in rheumatoid arthritis evaluated by C3dg and the terminal complement complex. Arthritis Rheum. 1986 Jun;29(6):715–721. doi: 10.1002/art.1780290603. [DOI] [PubMed] [Google Scholar]
- Mollnes T. E., Paus A. Complement activation in synovial fluid and tissue from patients with juvenile rheumatoid arthritis. Arthritis Rheum. 1986 Nov;29(11):1359–1364. doi: 10.1002/art.1780291108. [DOI] [PubMed] [Google Scholar]
- Mollnes T. E., Vandvik B., Lea T., Vartdal F. Intrathecal complement activation in neurological diseases evaluated by analysis of the terminal complement complex. J Neurol Sci. 1987 Mar;78(1):17–28. doi: 10.1016/0022-510x(87)90074-8. [DOI] [PubMed] [Google Scholar]
- Morgan B. P., Campbell A. K., Compston D. A. Terminal component of complement (C9) in cerebrospinal fluid of patients with multiple sclerosis. Lancet. 1984 Aug 4;2(8397):251–254. doi: 10.1016/s0140-6736(84)90298-8. [DOI] [PubMed] [Google Scholar]
- Niculescu F., Hugo F., Rus H. G., Vlaicu R., Bhakdi S. Quantitative evaluation of the terminal C5b-9 complement complex by ELISA in human atherosclerotic arteries. Clin Exp Immunol. 1987 Aug;69(2):477–483. [PMC free article] [PubMed] [Google Scholar]
- Podack E. R., Müller-Eberhard H. J. Binding of desoxycholate, phosphatidylcholine vesicles, lipoprotein and of the S-protein to complexes of terminal complement components. J Immunol. 1978 Sep;121(3):1025–1030. [PubMed] [Google Scholar]
- Rodbard D., McClean S. W. Automated computer analysis for enzyme-multiplied immunological techniques. Clin Chem. 1977 Jan;23(1):112–115. [PubMed] [Google Scholar]
- Salama A., Hugo F., Heinrich D., Höge R., Müller R., Kiefel V., Mueller-Eckhardt C., Bhakdi S. Deposition of terminal C5b-9 complement complexes on erythrocytes and leukocytes during cardiopulmonary bypass. N Engl J Med. 1988 Feb 18;318(7):408–414. doi: 10.1056/NEJM198802183180704. [DOI] [PubMed] [Google Scholar]
- Sanders M. E., Alexander E. L., Koski C. L., Frank M. M., Joiner K. A. Detection of activated terminal complement (C5b-9) in cerebrospinal fluid from patients with central nervous system involvement of primary Sjogren's syndrome or systemic lupus erythematosus. J Immunol. 1987 Apr 1;138(7):2095–2099. [PubMed] [Google Scholar]
- Sanders M. E., Koski C. L., Robbins D., Shin M. L., Frank M. M., Joiner K. A. Activated terminal complement in cerebrospinal fluid in Guillain-Barré syndrome and multiple sclerosis. J Immunol. 1986 Jun 15;136(12):4456–4459. [PubMed] [Google Scholar]
- Schäfer H., Mathey D., Hugo F., Bhakdi S. Deposition of the terminal C5b-9 complement complex in infarcted areas of human myocardium. J Immunol. 1986 Sep 15;137(6):1945–1949. [PubMed] [Google Scholar]
- Sliwinski A. J., Zvaifler N. J. Decreased synthesis of the third component of complement (C3) in hypocomplementemic systemic lupus erythematosus. Clin Exp Immunol. 1972 May;11(1):21–29. [PMC free article] [PubMed] [Google Scholar]
- Webster R. O., Larsen G. L., Henson P. M. In vivo clearance and tissue distribution of C5a and C5a des arginine complement fragments in rabbits. J Clin Invest. 1982 Dec;70(6):1177–1183. doi: 10.1172/JCI110716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisdorf D. J., Hammerschmidt D. E., Jacob H. S., Craddock P. R. Rapid in vivo clearance of C5ades arg: a possible protective mechanism against complement-mediated tissue injury. J Lab Clin Med. 1981 Dec;98(6):823–830. [PubMed] [Google Scholar]


