Abstract
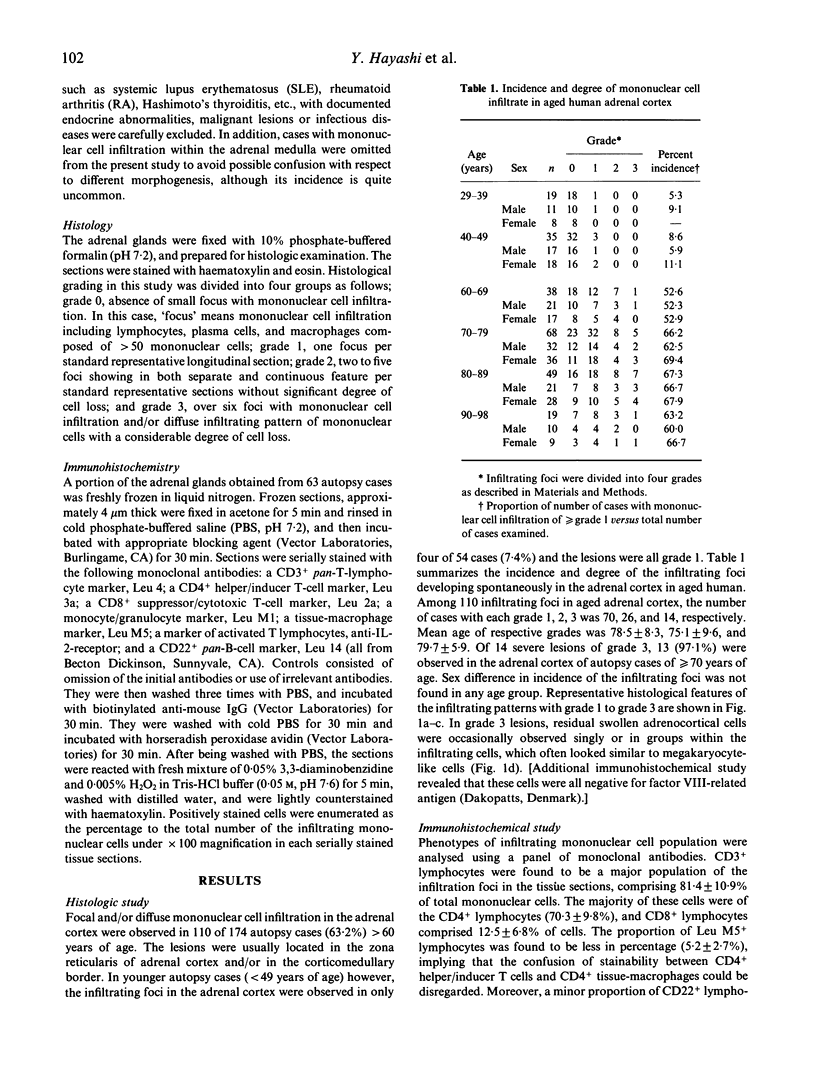
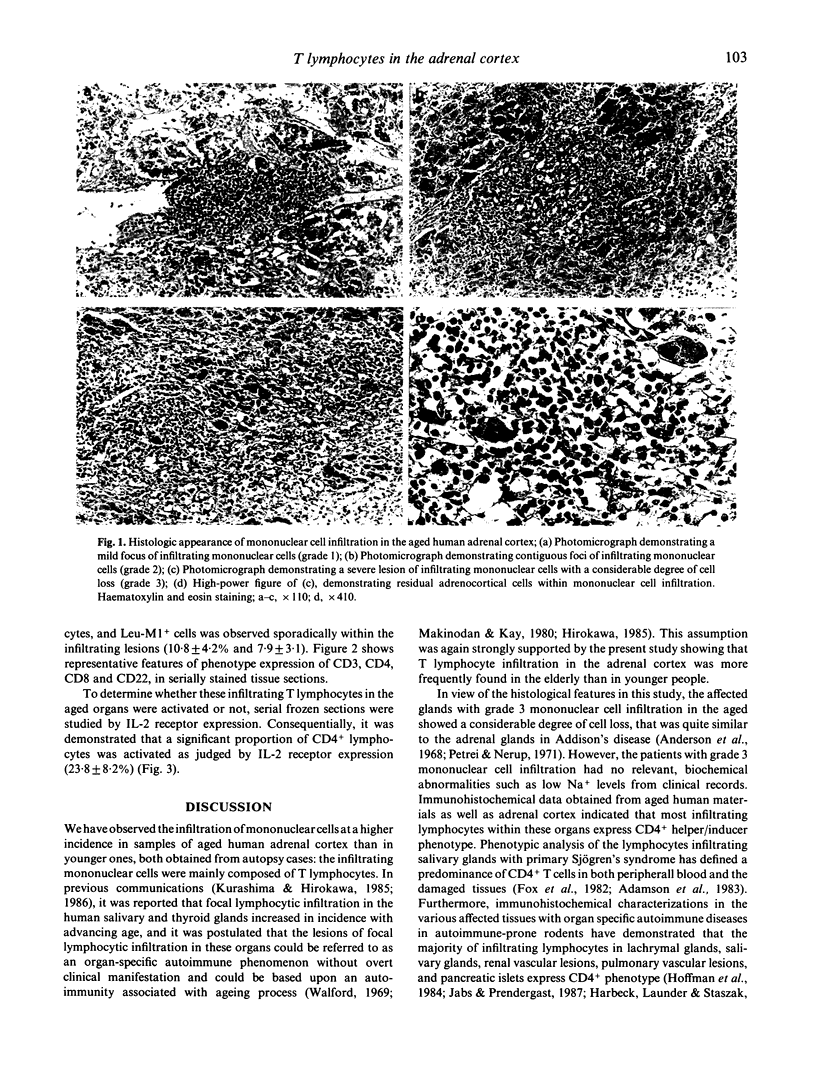
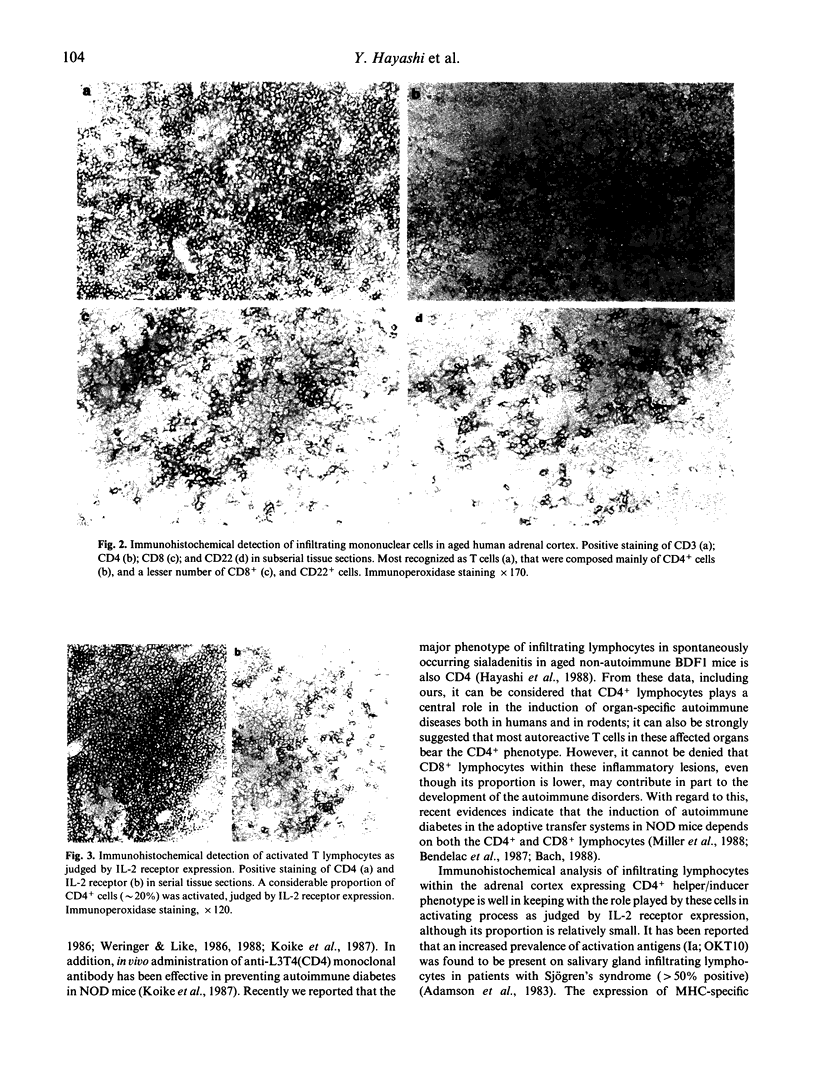
The incidence of mononuclear cell infiltration in the adrenal cortex was examined in autopsy cases of young and old subjects, and the infiltrating mononuclear cells were immunohistologically characterized by monoclonal antibodies. Histologically, 110 of 174 autopsy cases of persons greater than 60 years (63.2%) were shown to have mononuclear cell infiltration of varying degree within the adrenal cortex, whereas such a lesion was observed in lesser incidence (7.4%) in the 54 younger, control subjects aged less than 49 years. In addition, severely infiltrating lesions in the adrenal cortex were found frequently in the elderly greater than 70 years. Immunohistochemical study revealed that the infiltrating mononuclear cells were mainly composed of CD3+ T cells. The major proportion of CD3+ T cells expressed CD4, whereas CD8+ T cells were less in number. Moreover, a considerable proportion of CD4+ T cells was activated as judged by interleukin 2 receptor expression. These findings indicate that T lymphocytes infiltration in aged human adrenal cortex may represent a pre-clinical manifestation of organ-specific autoimmune adrenalitis which is based on autoimmunity associated with ageing process.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adamson T. C., 3rd, Fox R. I., Frisman D. M., Howell F. V. Immunohistologic analysis of lymphoid infiltrates in primary Sjogren's syndrome using monoclonal antibodies. J Immunol. 1983 Jan;130(1):203–208. [PubMed] [Google Scholar]
- Anderson J. R., Goudie R. B., Gray K., Stuart-Smith D. A. Immunological features of idiopathic Addison's disease: an antibody to cells producing steroid hormones. Clin Exp Immunol. 1968 Feb;3(2):107–117. [PMC free article] [PubMed] [Google Scholar]
- Bach J. F. Mechanisms of autoimmunity in insulin-dependent diabetes mellitus. Clin Exp Immunol. 1988 Apr;72(1):1–8. [PMC free article] [PubMed] [Google Scholar]
- Bendelac A., Carnaud C., Boitard C., Bach J. F. Syngeneic transfer of autoimmune diabetes from diabetic NOD mice to healthy neonates. Requirement for both L3T4+ and Lyt-2+ T cells. J Exp Med. 1987 Oct 1;166(4):823–832. doi: 10.1084/jem.166.4.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox R. I., Carstens S. A., Fong S., Robinson C. A., Howell F., Vaughan J. H. Use of monoclonal antibodies to analyze peripheral blood and salivary gland lymphocyte subsets in Sjögren's syndrome. Arthritis Rheum. 1982 Apr;25(4):419–426. doi: 10.1002/art.1780250410. [DOI] [PubMed] [Google Scholar]
- Harbeck R. J., Launder T., Staszak C. Mononuclear cell pulmonary vasculitis in NZB/W mice. II. Immunohistochemical characterization of the infiltrating cells. Am J Pathol. 1986 May;123(2):204–211. [PMC free article] [PubMed] [Google Scholar]
- Hayashi Y., Kurashima C., Utsuyama M., Hirokawa K. Spontaneous development of autoimmune sialadenitis in aging BDF1 mice. Am J Pathol. 1988 Jul;132(1):173–179. [PMC free article] [PubMed] [Google Scholar]
- Hoffman R. W., Alspaugh M. A., Waggie K. S., Durham J. B., Walker S. E. Sjögren's syndrome in MRL/l and MRL/n mice. Arthritis Rheum. 1984 Feb;27(2):157–165. doi: 10.1002/art.1780270206. [DOI] [PubMed] [Google Scholar]
- Jabs D. A., Prendergast R. A. Reactive lymphocytes in lacrimal gland and vasculitic renal lesions of autoimmune MRL/lpr mice express L3T4. J Exp Med. 1987 Oct 1;166(4):1198–1203. doi: 10.1084/jem.166.4.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koike T., Itoh Y., Ishii T., Ito I., Takabayashi K., Maruyama N., Tomioka H., Yoshida S. Preventive effect of monoclonal anti-L3T4 antibody on development of diabetes in NOD mice. Diabetes. 1987 Apr;36(4):539–541. doi: 10.2337/diab.36.4.539. [DOI] [PubMed] [Google Scholar]
- Kurashima C., Hirokawa K. Age-related increase of focal lymphocytic infiltration in the human submandibular glands. J Oral Pathol. 1986 Mar;15(3):172–178. doi: 10.1111/j.1600-0714.1986.tb00601.x. [DOI] [PubMed] [Google Scholar]
- Kurashima C., Hirokawa K. Focal lymphocytic infiltration in thyroids of elderly people. Histopathological and immunohistochemical studies. Surv Synth Pathol Res. 1985;4(5-6):457–466. doi: 10.1159/000156996. [DOI] [PubMed] [Google Scholar]
- Makinodan T., Kay M. M. Age influence on the immune system. Adv Immunol. 1980;29:287–330. doi: 10.1016/s0065-2776(08)60047-4. [DOI] [PubMed] [Google Scholar]
- Miller B. J., Appel M. C., O'Neil J. J., Wicker L. S. Both the Lyt-2+ and L3T4+ T cell subsets are required for the transfer of diabetes in nonobese diabetic mice. J Immunol. 1988 Jan 1;140(1):52–58. [PubMed] [Google Scholar]
- Petri M., Nerup J. Addison's adrenalitis. Studies on diffuse lymphocytic adrenalitis (idiopathic Addison's disease) and focal lymphocytic infiltration in a control material. Acta Pathol Microbiol Scand A. 1971;79(4):381–388. [PubMed] [Google Scholar]
- Weringer E. J., Like A. A. Identification of T cell subsets and class I and class II antigen expression in islet grafts and pancreatic islets of diabetic BioBreeding/Worcester rats. Am J Pathol. 1988 Aug;132(2):292–303. [PMC free article] [PubMed] [Google Scholar]