Abstract
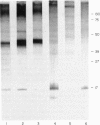
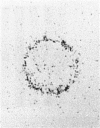
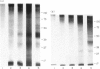
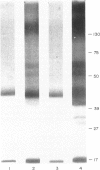
Various methods of radioiodination were compared for their efficacy in labelling the surface of Strongyloides ratti infective larvae and adult worms. The Iodogen method was chosen as the optimal technique for this parasite. The surface location of 125-iodine was confirmed with light microscope autoradiography of transverse sections of labelled worms. Stage-specific surface components were identified when the sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE) profiles of infective larvae and adult worms were compared. Labelled surface molecules were solubilized with either the non-ionic detergent Triton-X-100, the anionic detergents sodium deoxycholate (DOC) or sodium dodecyl sulphate (SDS), or the cationic detergent cetyl trimethyl-ammonium bromide (CTAB). The CTAB extract yielded most labelled proteins that retained their antigenicity in an immunoprecipitation assay with hyperimmune mouse sera. Immunoprecipitation analysis with stage-specific mouse sera revealed that the surface of infective larvae is immunogenic and that there are no cross-reactions with adult worms. Adult worms resident in the intestine were not found to be immunogenic and showed a complete absence of reactivity. Antigenic determinants shared between S. ratti and S. stercoralis were identified. Patients infected with S. stercoralis precipitated bands with molecular weights 32 and 34 kD which were not reactive with normal sera. These reactions suggest the potential usefulness of the surface of S. ratti as a source of diagnostic antigens.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Betschart B., Jenkins J. M. Distribution of iodinated proteins in Dipetalonema viteae after surface labelling. Mol Biochem Parasitol. 1987 Jan 2;22(1):1–8. doi: 10.1016/0166-6851(87)90063-6. [DOI] [PubMed] [Google Scholar]
- Bolton A. E., Hunter W. M. The labelling of proteins to high specific radioactivities by conjugation to a 125I-containing acylating agent. Biochem J. 1973 Jul;133(3):529–539. doi: 10.1042/bj1330529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll S. M., Karthigasu K. T., Grove D. I. Serodiagnosis of human strongyloidiasis by an enzyme-linked immunosorbent assay. Trans R Soc Trop Med Hyg. 1981;75(5):706–709. doi: 10.1016/0035-9203(81)90156-5. [DOI] [PubMed] [Google Scholar]
- Dawkins H. J., Grove D. I., Dunsmore J. D., Mitchell G. F. Strongyloides ratti: susceptibility to infection and resistance to reinfection in inbred strains of mice as assessed by excretion of larvae. Int J Parasitol. 1980 Apr;10(2):125–129. doi: 10.1016/0020-7519(80)90023-5. [DOI] [PubMed] [Google Scholar]
- Fraker P. J., Speck J. C., Jr Protein and cell membrane iodinations with a sparingly soluble chloroamide, 1,3,4,6-tetrachloro-3a,6a-diphrenylglycoluril. Biochem Biophys Res Commun. 1978 Feb 28;80(4):849–857. doi: 10.1016/0006-291x(78)91322-0. [DOI] [PubMed] [Google Scholar]
- Grove D. I., Northern C. Oral transfer of Strongyloides ratti adult worms to mice. J Parasitol. 1987 Apr;73(2):424–425. [PubMed] [Google Scholar]
- HUNTER W. M., GREENWOOD F. C. Preparation of iodine-131 labelled human growth hormone of high specific activity. Nature. 1962 May 5;194:495–496. doi: 10.1038/194495a0. [DOI] [PubMed] [Google Scholar]
- Hubbard A. L., Cohn Z. A. Externally disposed plasma membrane proteins. I. Enzymatic iodination of mouse L cells. J Cell Biol. 1975 Feb;64(2):438–460. doi: 10.1083/jcb.64.2.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall E., Howells R. E. An evaluation of different methods for labelling the surface of the filarial nematode Brugia pahangi with 125iodine. Mol Biochem Parasitol. 1985 Jun;15(3):295–304. doi: 10.1016/0166-6851(85)90091-x. [DOI] [PubMed] [Google Scholar]
- Murrell K. D., Graham C. E. Shedding of antibody complexes by Strongyloides ratti (Nematoda) larvae. J Parasitol. 1983 Feb;69(1):70–73. [PubMed] [Google Scholar]
- Northern C., Grove D. I. Antigenic analysis of Strongyloides ratti infective larvae and adult worms. Immunol Cell Biol. 1987 Jun;65(Pt 3):231–239. doi: 10.1038/icb.1987.26. [DOI] [PubMed] [Google Scholar]
- Northern C., Grove D. I. Western blot analysis of reactivity to larval and adult Strongyloides ratti antigens in mice. Parasite Immunol. 1988 Nov;10(6):681–691. doi: 10.1111/j.1365-3024.1988.tb00254.x. [DOI] [PubMed] [Google Scholar]
- Philipp M., Rumjaneck F. D. Antigenic and dynamic properties of helminth surface structures. Mol Biochem Parasitol. 1984 Mar;10(3):245–268. doi: 10.1016/0166-6851(84)90025-2. [DOI] [PubMed] [Google Scholar]
- Pritchard D. I., Crawford C. R., Duce I. R., Behnke J. M. Antigen stripping from the nematode epicuticle using the cationic detergent cetyltrimethylammonium bromide (CTAB). Parasite Immunol. 1985 Nov;7(6):575–585. doi: 10.1111/j.1365-3024.1985.tb00101.x. [DOI] [PubMed] [Google Scholar]