Abstract
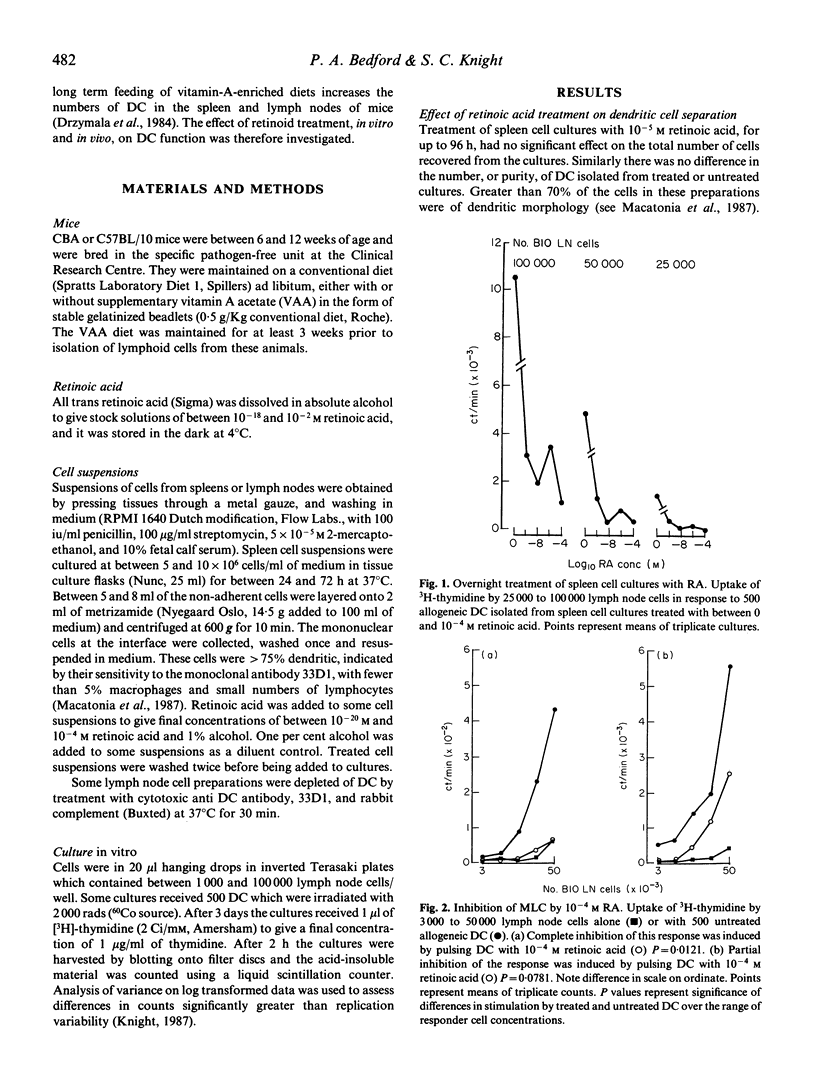
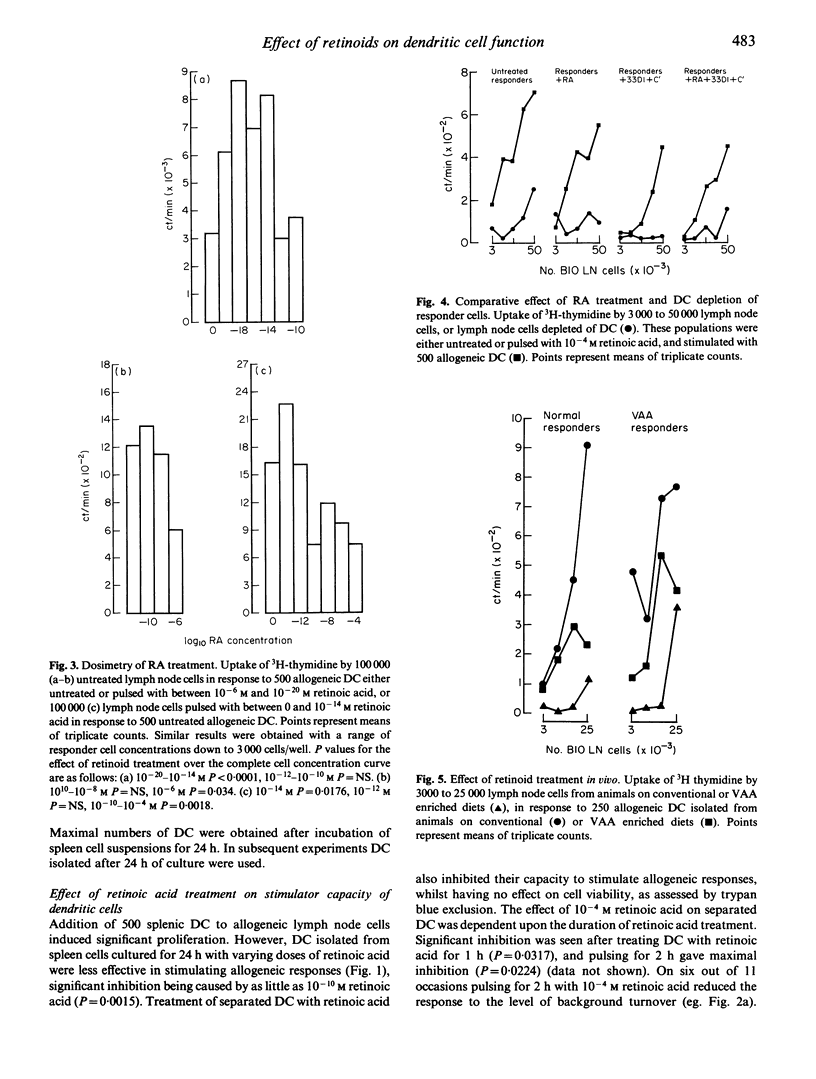
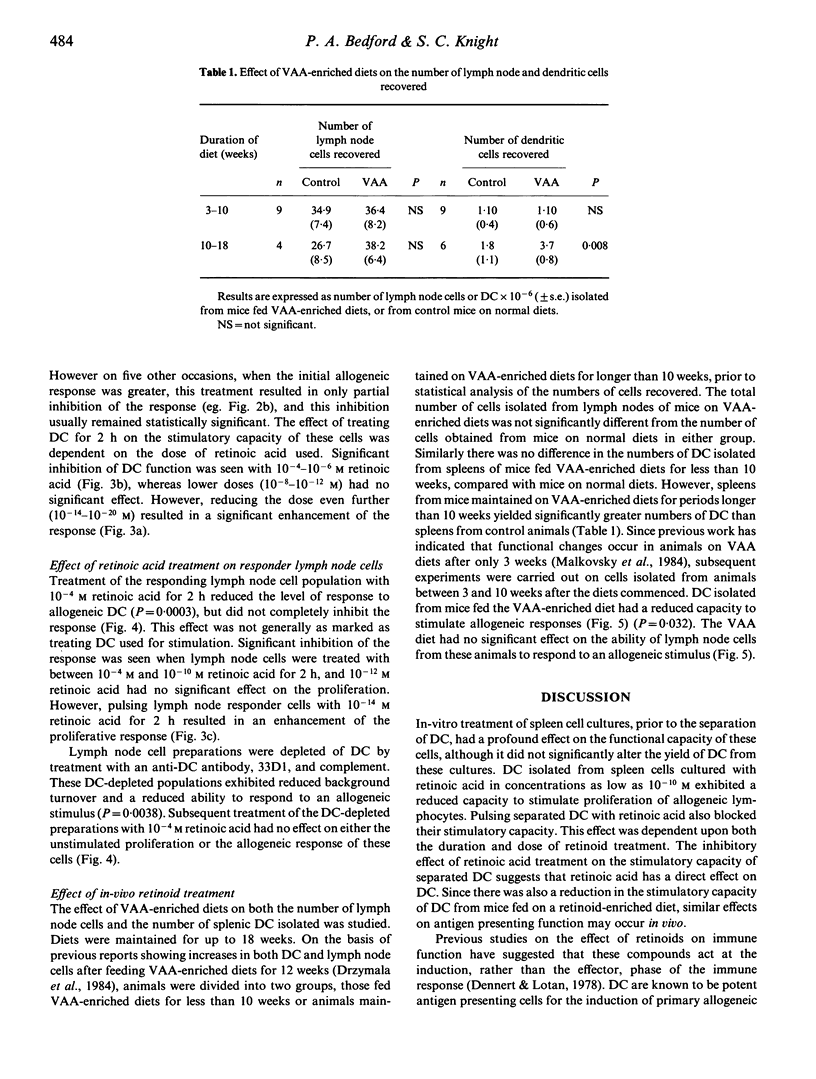
The effect of retinoid administration on the antigen presenting function of mouse dendritic cells (DC) was assessed. Culturing spleen cells with retinoic acid (10(-10)-10(-4) M) had no effect on the numbers of DC separated from these cultures. However, DC isolated from retinoic-acid-treated cultures were less stimulatory than DC from untreated cultures when added to allogeneic lymphocytes in a mixed leucocyte culture. Allogeneic stimulation by DC was also inhibited by pulsing separated DC with retinoic acid (10(-6)-10(-4) M) for 2 h. Pulsing DC with lower doses of retinoic acid (10(-14)-10(-20) M) enhanced this response. DC isolated from animals maintained on VAA-enriched diets had a reduced capacity to stimulate allogeneic lymphocytes. The response of unseparated lymph node cells pulsed with retinoic acid to untreated allogeneic DC was inhibited by 10(-10)-10(-4) M retinoic acid but enhanced by lower doses (10(-14) M). However, the inhibitory effect of retinoids on the function of responding lymphoid populations was abolished on removal of DC from responding cells. The results indicate that immunomodulation by retinoids could occur via an effect on the efficiency of antigen presentation by DC.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnett J. B. Immunomodulating effects of 13-cis retinoic acid on the IgG and IgM response of BALB/c mice. Int Arch Allergy Appl Immunol. 1983;72(3):227–233. doi: 10.1159/000234872. [DOI] [PubMed] [Google Scholar]
- Bauer R., Orfanos C. E. Trimethylmethoxyphenyl-retinoic acid (Ro 10-1670) inhibits mitogen-induced DNA-synthesis in peripheral blood lymphocytes in vitro. Br J Dermatol. 1981 Jul;105(1):19–24. doi: 10.1111/j.1365-2133.1981.tb00878.x. [DOI] [PubMed] [Google Scholar]
- Colizzi V., Malkovsky M. Augmentation of interleukin-2 production and delayed hypersensitivity in mice infected with Mycobacterium bovis and fed a diet supplemented with vitamin A acetate. Infect Immun. 1985 May;48(2):581–583. doi: 10.1128/iai.48.2.581-583.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennert G., Lotan R. Effects of retinoic acid on the immune system: stimulation of T killer cell induction. Eur J Immunol. 1978 Jan;8(1):23–29. doi: 10.1002/eji.1830080106. [DOI] [PubMed] [Google Scholar]
- Dillehay D. L., Li W., Kalin J., Walia A. S., Lamon E. W. In vitro effects of retinoids on murine thymus-dependent and thymus-independent mitogenesis. Cell Immunol. 1987 Jun;107(1):130–137. doi: 10.1016/0008-8749(87)90273-5. [DOI] [PubMed] [Google Scholar]
- Floersheim G. L., Bollag W. Accelerated rejection of skin homografts by vitamin A acid. Transplantation. 1972 Nov;14(5):564–567. doi: 10.1097/00007890-197211000-00006. [DOI] [PubMed] [Google Scholar]
- Haftek M., Faure M., Schmitt D., Thivolet J. Langerhans cells in skin from patients with psoriasis: quantitative and qualitative study of T6 and HLA-DR antigen-expressing cells and changes with aromatic retinoid administration. J Invest Dermatol. 1983 Jul;81(1):10–14. doi: 10.1111/1523-1747.ep12537454. [DOI] [PubMed] [Google Scholar]
- Jurin M., Tannock I. F. Influence of vitamin A on immunological response. Immunology. 1972 Sep;23(3):283–287. [PMC free article] [PubMed] [Google Scholar]
- Lotan R. Effects of vitamin A and its analogs (retinoids) on normal and neoplastic cells. Biochim Biophys Acta. 1980 Mar 12;605(1):33–91. doi: 10.1016/0304-419x(80)90021-9. [DOI] [PubMed] [Google Scholar]
- Macatonia S. E., Knight S. C., Edwards A. J., Griffiths S., Fryer P. Localization of antigen on lymph node dendritic cells after exposure to the contact sensitizer fluorescein isothiocyanate. Functional and morphological studies. J Exp Med. 1987 Dec 1;166(6):1654–1667. doi: 10.1084/jem.166.6.1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malkovský M., Edwards A. J., Hunt R., Palmer L., Medawar P. B. T-cell-mediated enhancement of host-versus-graft reactivity in mice fed a diet enriched in vitamin A acetate. Nature. 1983 Mar 24;302(5906):338–340. doi: 10.1038/302338a0. [DOI] [PubMed] [Google Scholar]
- Malkovský M., Hunt R., Palmer L., Doré C., Medawar P. B. Retinyl acetate-mediated augmentation of resistance to a transplantable 3-methylcholanthrene-induced fibrosarcoma. The dose response and time course. Transplantation. 1984 Aug;38(2):158–161. doi: 10.1097/00007890-198408000-00013. [DOI] [PubMed] [Google Scholar]
- Malkovský M., Medawar P. B., Thatcher D. R., Toy J., Hunt R., Rayfield L. S., Doré C. Acquired immunological tolerance of foreign cells is impaired by recombinant interleukin 2 or vitamin A acetate. Proc Natl Acad Sci U S A. 1985 Jan;82(2):536–538. doi: 10.1073/pnas.82.2.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micksche M., Cerni C., Kokron O., Titscher R., Wrba H. Stimulation of immune response in lung cancer patients by vitamin A therapy. Oncology. 1977;34(5):234–238. doi: 10.1159/000225231. [DOI] [PubMed] [Google Scholar]
- Miller K., Maisey J., Malkovský M. Enhancement of contact sensitization in mice fed a diet enriched in vitamin A acetate. Int Arch Allergy Appl Immunol. 1984;75(2):120–125. doi: 10.1159/000233601. [DOI] [PubMed] [Google Scholar]
- Orfanos C. E., Bauer R. Evidence for anti-inflammatory activities of oral synthetic retinoids: experimental findings and clinical experience. Br J Dermatol. 1983 Jul;109 (Suppl 25):55–60. [PubMed] [Google Scholar]
- Rhodes J., Oliver S. Retinoids as regulators of macrophage function. Immunology. 1980 Jul;40(3):467–472. [PMC free article] [PubMed] [Google Scholar]
- Sherwood R. A., Brent L., Rayfield L. S. Presentation of alloantigens by host cells. Eur J Immunol. 1986 May;16(5):569–574. doi: 10.1002/eji.1830160519. [DOI] [PubMed] [Google Scholar]
- Sontheimer R. D., Bergstresser P. R., Gailiunas P., Jr, Helderman J. H., Gilliam J. N. Perturbation of epidermal Langerhans cells in immunosuppressed human renal allograft recipients. Transplantation. 1984 Feb;37(2):168–174. doi: 10.1097/00007890-198402000-00011. [DOI] [PubMed] [Google Scholar]
- Steinman R. M., Witmer M. D. Lymphoid dendritic cells are potent stimulators of the primary mixed leukocyte reaction in mice. Proc Natl Acad Sci U S A. 1978 Oct;75(10):5132–5136. doi: 10.1073/pnas.75.10.5132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turton J. A., Hicks R. M., Gwynne J., Hunt R., Hawkey C. M. Retinoid toxicity. Ciba Found Symp. 1985;113:220–251. doi: 10.1002/9780470720943.ch13. [DOI] [PubMed] [Google Scholar]
- Walsh L. J., Seymour G. J., Powell R. N. The in vitro effect of retinol on human gingival epithelium. II. Modulation of Langerhans cell markers and interleukin-1 production. J Invest Dermatol. 1985 Dec;85(6):501–506. doi: 10.1111/1523-1747.ep12277300. [DOI] [PubMed] [Google Scholar]


