Abstract
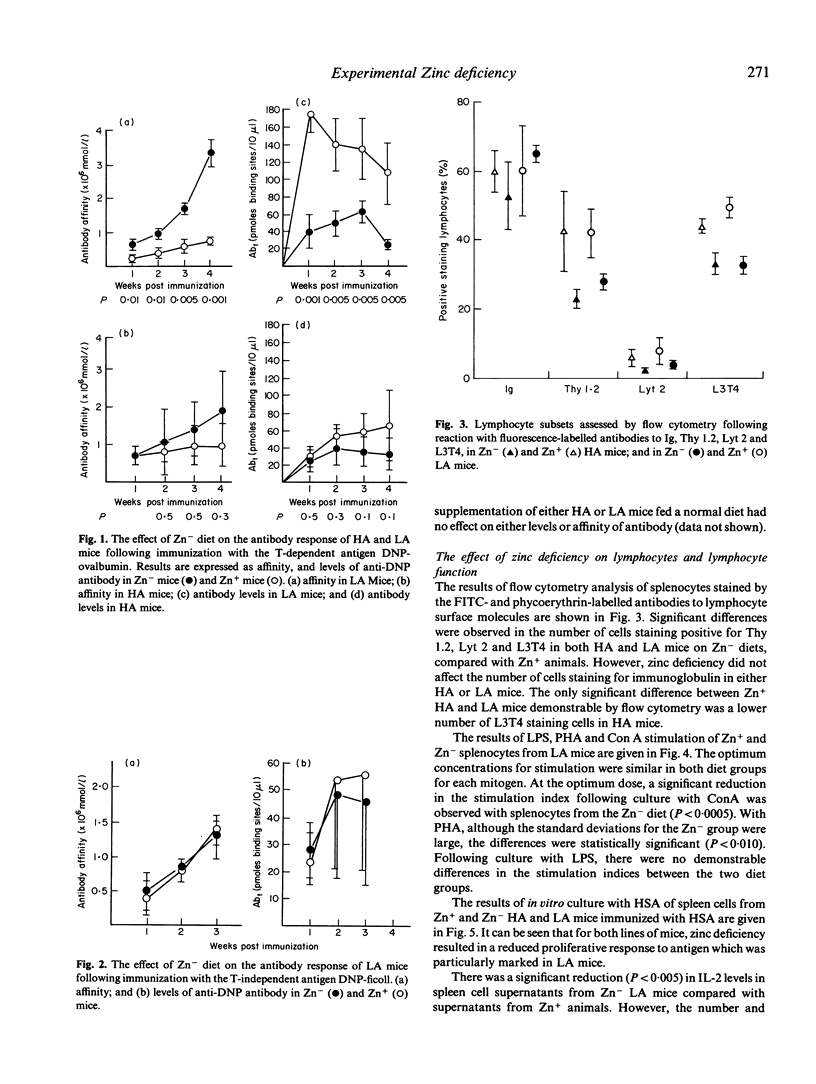
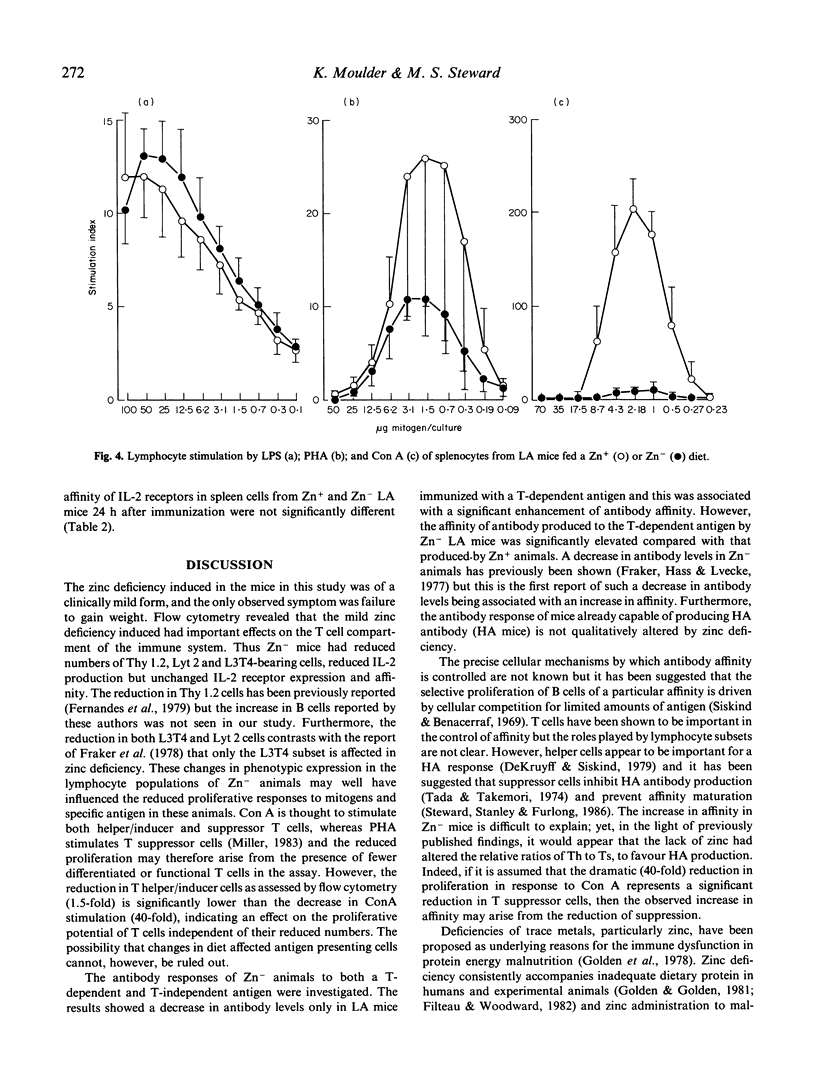
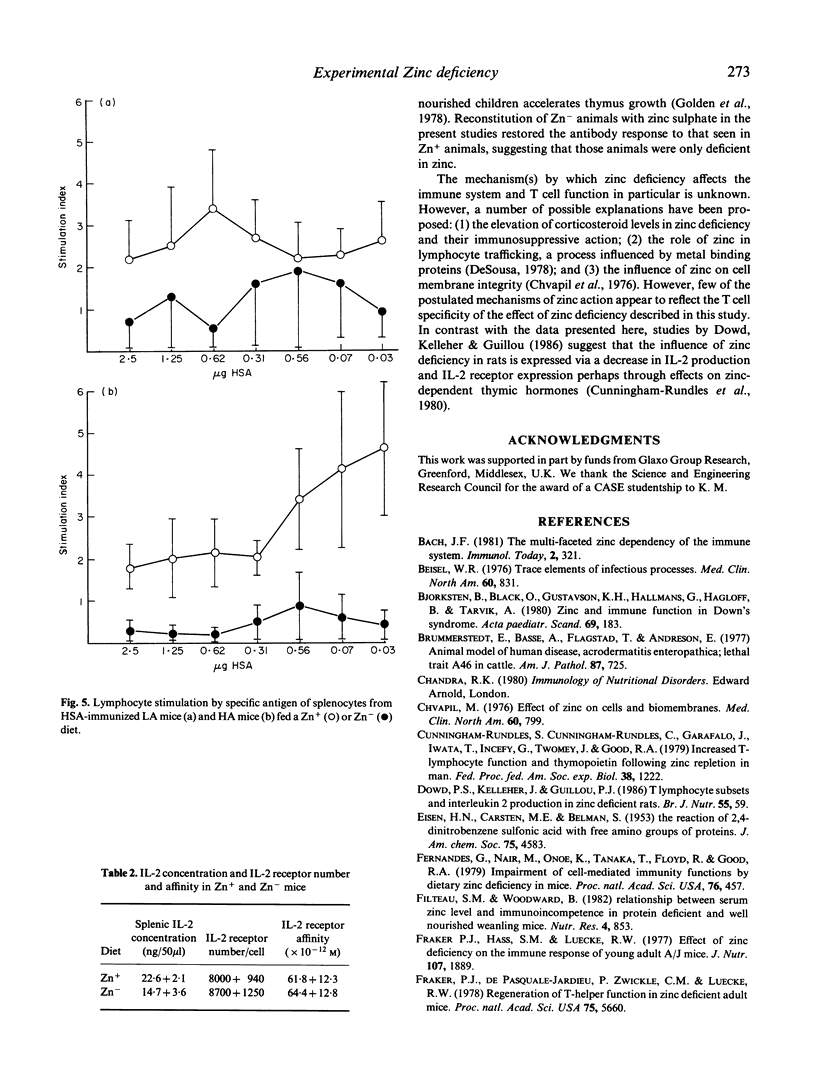
Mice genetically selected for the production of either high- or low-affinity antibody were fed on diets sufficient or deficient in zinc. The effect of zinc deficiency on immune responses in these animals was analysed in terms of cell-mediated responses and the levels and affinity of antibody produced in response to immunization with T-dependent and T-independent antigens. In comparison with animals fed zinc-containing diets, mice fed zinc-deficient diets had reduced numbers of T cells and T-cell subsets, reduced proliferation to mitogens and specific antigen, and a decreased production of interleukin 2 (IL-2), but the number and affinity of IL-2 receptors were not affected. Furthermore, zinc-deficient animals produced reduced levels of antibody to the T-dependent antigen DNP-human serum albumin, but the affinity of this antibody was significantly elevated compared with that produced by zinc-sufficient animals. However, zinc deficiency had no effect on the levels and affinity of antibody produced to the T-independent antigen DNP-ficoll.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beisel W. R. Trace element in infectious processes. Med Clin North Am. 1976 Jul;60(4):831–849. doi: 10.1016/s0025-7125(16)31864-8. [DOI] [PubMed] [Google Scholar]
- Björkstén B., Bäck O., Gustavson K. H., Hallmans G., Hägglöf B., Tärnvik A. Zinc and immune function in Down's syndrome. Acta Paediatr Scand. 1980 Mar;69(2):183–187. doi: 10.1111/j.1651-2227.1980.tb07057.x. [DOI] [PubMed] [Google Scholar]
- Brummerstedt E. Animal model of human disease. Acrodermatitis enteropathica, zinc malabsorption. Am J Pathol. 1977 Jun;87(3):725–728. [PMC free article] [PubMed] [Google Scholar]
- Chvapil M. Effect of zinc on cells and biomembranes. Med Clin North Am. 1976 Jul;60(4):799–812. [PubMed] [Google Scholar]
- De Sousa M. Lymphoid cell positioning: a new proposal for the mechanism of control of lymphoid cell migration. Symp Soc Exp Biol. 1978;32:393–410. [PubMed] [Google Scholar]
- DeKruyff R., Siskind G. W. Studies on the control of antibody synthesis. XIV. Role of T cells in regulating antibody affinity. Cell Immunol. 1979 Sep 15;47(1):134–142. doi: 10.1016/0008-8749(79)90321-6. [DOI] [PubMed] [Google Scholar]
- Dowd P. S., Kelleher J., Guillou P. J. T-lymphocyte subsets and interleukin-2 production in zinc-deficient rats. Br J Nutr. 1986 Jan;55(1):59–69. doi: 10.1079/bjn19860010. [DOI] [PubMed] [Google Scholar]
- Fernandes G., Nair M., Onoe K., Tanaka T., Floyd R., Good R. A. Impairment of cell-mediated immunity functions by dietary zinc deficiency in mice. Proc Natl Acad Sci U S A. 1979 Jan;76(1):457–461. doi: 10.1073/pnas.76.1.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filteau S. M., Woodward B. The effect of severe protein deficiency on serum zinc concentration of mice fed a requirement level or a very high level of dietary zinc. J Nutr. 1982 Oct;112(10):1974–1977. doi: 10.1093/jn/112.10.1974. [DOI] [PubMed] [Google Scholar]
- Fraker P. J., DePasquale-Jardieu P., Zwickl C. M., Luecke R. W. Regeneration of T-cell helper function in zinc-deficient adult mice. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5660–5664. doi: 10.1073/pnas.75.11.5660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraker P. J., Haas S. M., Luecke R. W. Effect of zinc deficiency on the immune response of the young adult A/J mouse. J Nutr. 1977 Oct;107(10):1889–1895. doi: 10.1093/jn/107.10.1889. [DOI] [PubMed] [Google Scholar]
- Gershon R. K., Paul W. E. Effect of thymus-derived lymphocytes on amount and affinity of anti-hapten antibody. J Immunol. 1971 Mar;106(3):872–874. [PubMed] [Google Scholar]
- Golden M. H., Golden B. E. Effect of zinc supplementation on the dietary intake, rate of weight gain, and energy cost of tissue deposition in children recovering from severe malnutrition. Am J Clin Nutr. 1981 May;34(5):900–908. doi: 10.1093/ajcn/34.5.900. [DOI] [PubMed] [Google Scholar]
- Golden M. H., Harland P. S., Golden B. E., Jackson A. A. Zinc and immunocompetence in protein-energy malnutrition. Lancet. 1978 Jun 10;1(8076):1226–1228. doi: 10.1016/s0140-6736(78)92463-7. [DOI] [PubMed] [Google Scholar]
- Homsy J., Morrow W. J., Levy J. A. Nutrition and autoimmunity: a review. Clin Exp Immunol. 1986 Sep;65(3):473–488. [PMC free article] [PubMed] [Google Scholar]
- Inman J. K. Thymus-independent antigens: the preparation of covalent, hapten-ficoll conjugates. J Immunol. 1975 Feb;114(2 Pt 1):704–709. [PubMed] [Google Scholar]
- Iwata T., Incefy G. S., Tanaka T., Fernandes G., Menendez-Botet C. J., Pih K., Good R. A. Circulating thymic hormone levels in zinc deficiency. Cell Immunol. 1979 Sep 15;47(1):100–105. doi: 10.1016/0008-8749(79)90318-6. [DOI] [PubMed] [Google Scholar]
- Katz F. E., Steward M. W. The genetic control of antibody affinity in mice. Immunology. 1975 Sep;29(3):543–548. [PMC free article] [PubMed] [Google Scholar]
- Lennard E. S., Bjornson A. B., Petering H. G., Alexander J. W. An immunologic and nutritional evaluation of burn neutrophil function. J Surg Res. 1974 Mar;16(3):286–298. doi: 10.1016/0022-4804(74)90045-6. [DOI] [PubMed] [Google Scholar]
- Miller R. A. IL 2 production by mitogen-stimulated T cell subsets: helper-precursors are predominantly Lyt-2-. J Immunol. 1983 Dec;131(6):2864–2867. [PubMed] [Google Scholar]
- Oleske J. M., Westphal M. L., Shore S., Gorden D., Bogden J. D., Nahmias A. Zinc therapy of depressed cellular immunity in acrodermatitis enteropathica. Its correction. Am J Dis Child. 1979 Sep;133(9):915–918. doi: 10.1001/archpedi.1979.02130090043007. [DOI] [PubMed] [Google Scholar]
- Siskind G. W., Benacerraf B. Cell selection by antigen in the immune response. Adv Immunol. 1969;10:1–50. doi: 10.1016/s0065-2776(08)60414-9. [DOI] [PubMed] [Google Scholar]
- Stanley C., Lew A. M., Steward M. W. The measurement of antibody affinity: a comparison of five techniques utilizing a panel of monoclonal anti-DNP antibodies and the effect of high affinity antibody on the measurement of low affinity antibody. J Immunol Methods. 1983 Nov 11;64(1-2):119–132. doi: 10.1016/0022-1759(83)90390-3. [DOI] [PubMed] [Google Scholar]
- Steward M. W. Chronic immune complex disease in mice: the role of antibody affinity. Clin Exp Immunol. 1979 Dec;38(3):414–423. [PMC free article] [PubMed] [Google Scholar]
- Steward M. W., Stanley C., Furlong M. D. Antibody affinity maturation in selectively bred high and low-affinity mice. Eur J Immunol. 1986 Jan;16(1):59–63. doi: 10.1002/eji.1830160112. [DOI] [PubMed] [Google Scholar]
- Tada T., Takemori T. Selective roles of thymus-derived lymphocytes in the antibody response. I. Differential suppressive effect of carrier-primed T cells on hapten-specific IgM and IgG antibody responses. J Exp Med. 1974 Jul 1;140(1):239–252. doi: 10.1084/jem.140.1.239. [DOI] [PMC free article] [PubMed] [Google Scholar]



