Abstract
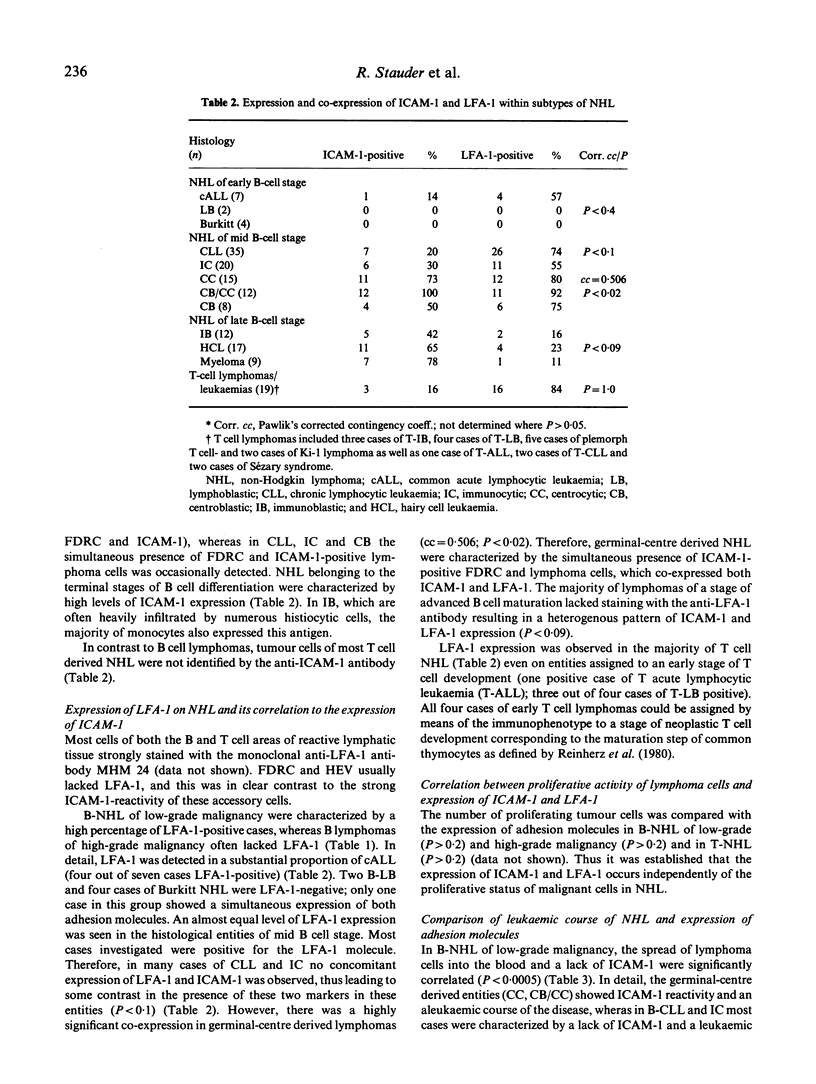
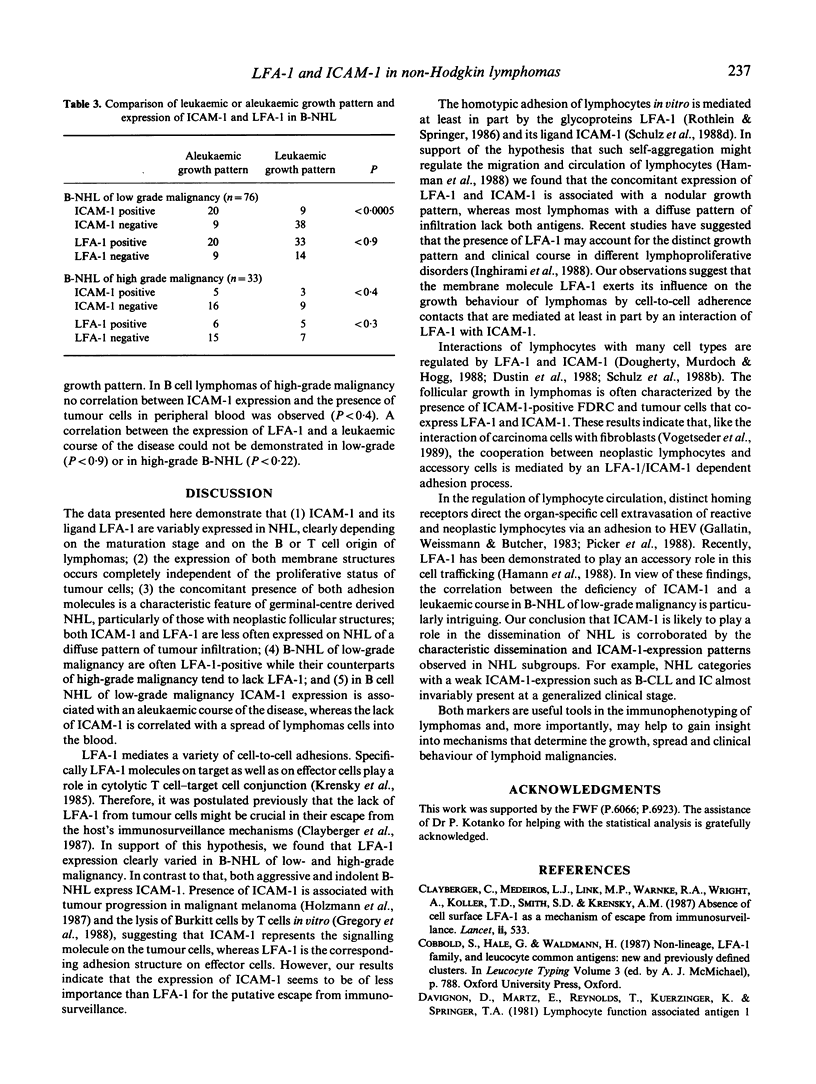
In 160 non-Hodgkin lymphomas (NHL) of B and T cell origin the expression of leucocyte function-associated antigen-1 (LFA-1) and one of its counter-structures named 7F7-antigen was studied. Functional and structural similarities and the 7F7-reactivity of pICAM-1 transfected COS-cells prove that 7F7-antigen is identical with ICAM-1. The expression of both adhesion structures occurred variably and clearly depended on the maturation stage and on the B and T cell origin of lymphomas but was not associated with the proliferative status of tumour cells. The concomitant expression of both adhesion molecules was a characteristic feature of germinal centre derived NHL, particularly of those with neoplastic follicular structures (corr. contingency coeff. = 0.506; P less than 0.02), whereas both adhesion structures were less often expressed on lymphomas with a more diffuse pattern of tumour infiltration. Most B-NHL of low-grade malignancy expressed LFA-1 while their counterparts of high-grade malignancy often tended to be LFA-1 negative. In B cell neoplasias of low-grade malignancy the lack of ICAM-1 and a leukaemic course of the disease were significantly correlated (P less than 0.0005). The results indicate that the differential expression of both adhesion molecules may account for distinct patterns of growth and spread in subtypes of lymphoid malignancies.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Clayberger C., Wright A., Medeiros L. J., Koller T. D., Link M. P., Smith S. D., Warnke R. A., Krensky A. M. Absence of cell surface LFA-1 as a mechanism of escape from immunosurveillance. Lancet. 1987 Sep 5;2(8558):533–536. doi: 10.1016/s0140-6736(87)92924-2. [DOI] [PubMed] [Google Scholar]
- Davignon D., Martz E., Reynolds T., Kürzinger K., Springer T. A. Lymphocyte function-associated antigen 1 (LFA-1): a surface antigen distinct from Lyt-2,3 that participates in T lymphocyte-mediated killing. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4535–4539. doi: 10.1073/pnas.78.7.4535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty G. J., Murdoch S., Hogg N. The function of human intercellular adhesion molecule-1 (ICAM-1) in the generation of an immune response. Eur J Immunol. 1988 Jan;18(1):35–39. doi: 10.1002/eji.1830180107. [DOI] [PubMed] [Google Scholar]
- Dustin M. L., Singer K. H., Tuck D. T., Springer T. A. Adhesion of T lymphoblasts to epidermal keratinocytes is regulated by interferon gamma and is mediated by intercellular adhesion molecule 1 (ICAM-1). J Exp Med. 1988 Apr 1;167(4):1323–1340. doi: 10.1084/jem.167.4.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallatin W. M., Weissman I. L., Butcher E. C. A cell-surface molecule involved in organ-specific homing of lymphocytes. Nature. 1983 Jul 7;304(5921):30–34. doi: 10.1038/304030a0. [DOI] [PubMed] [Google Scholar]
- Gattringer C., Huber H., Radaszkiewicz T., Pfaller W., Braunsteiner H. Imbalance of helper and suppressor T lymphocytes in malignant non-Hodgkin lymphomas: an in situ morphometric analysis. Int J Cancer. 1984 Jun 15;33(6):751–757. doi: 10.1002/ijc.2910330607. [DOI] [PubMed] [Google Scholar]
- Gerdes J., Schwab U., Lemke H., Stein H. Production of a mouse monoclonal antibody reactive with a human nuclear antigen associated with cell proliferation. Int J Cancer. 1983 Jan 15;31(1):13–20. doi: 10.1002/ijc.2910310104. [DOI] [PubMed] [Google Scholar]
- Gregory C. D., Murray R. J., Edwards C. F., Rickinson A. B. Downregulation of cell adhesion molecules LFA-3 and ICAM-1 in Epstein-Barr virus-positive Burkitt's lymphoma underlies tumor cell escape from virus-specific T cell surveillance. J Exp Med. 1988 Jun 1;167(6):1811–1824. doi: 10.1084/jem.167.6.1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greil K., Gattringer C., Fasching B., Cleveland J., Thaler J., Radaskiewicz T., Gastl G., Huber C., Rapp U., Huber H. abl oncogene expression in non-Hodgkin lymphomas: correlation to histological differentiation and clinical status. Int J Cancer. 1988 Oct 15;42(4):529–538. doi: 10.1002/ijc.2910420410. [DOI] [PubMed] [Google Scholar]
- Greil R., Gattringer C., Schulz T., Knapp W., Radaskiewicz T., Dierich M. P., Huber H. Receptors for the third component of complement: their association with maturation stage in non-Hodgkin lymphomas (NHL) and their possible implication with the development of follicular structures. Clin Exp Immunol. 1986 May;64(2):423–431. [PMC free article] [PubMed] [Google Scholar]
- Hildreth J. E., Gotch F. M., Hildreth P. D., McMichael A. J. A human lymphocyte-associated antigen involved in cell-mediated lympholysis. Eur J Immunol. 1983 Mar;13(3):202–208. doi: 10.1002/eji.1830130305. [DOI] [PubMed] [Google Scholar]
- Holzmann B., Bröcker E. B., Lehmann J. M., Ruiter D. J., Sorg C., Riethmüller G., Johnson J. P. Tumor progression in human malignant melanoma: five stages defined by their antigenic phenotypes. Int J Cancer. 1987 Apr 15;39(4):466–471. doi: 10.1002/ijc.2910390410. [DOI] [PubMed] [Google Scholar]
- Inghirami G., Wieczorek R., Zhu B. Y., Silber R., Dalla-Favera R., Knowles D. M. Differential expression of LFA-1 molecules in non-Hodgkin's lymphoma and lymphoid leukemia. Blood. 1988 Oct;72(4):1431–1434. [PubMed] [Google Scholar]
- Krensky A. M., Mentzer S. J., Clayberger C., Anderson D. C., Schmalstieg F. C., Burakoff S. J., Springer T. A. Heritable lymphocyte function-associated antigen-1 deficiency: abnormalities of cytotoxicity and proliferation associated with abnormal expression of LFA-1. J Immunol. 1985 Nov;135(5):3102–3108. [PubMed] [Google Scholar]
- Picker L. J., Medeiros L. J., Weiss L. M., Warnke R. A., Butcher E. C. Expression of lymphocyte homing receptor antigen in non-Hodgkin's lymphoma. Am J Pathol. 1988 Mar;130(3):496–504. [PMC free article] [PubMed] [Google Scholar]
- Reinherz E. L., Kung P. C., Goldstein G., Levey R. H., Schlossman S. F. Discrete stages of human intrathymic differentiation: analysis of normal thymocytes and leukemic lymphoblasts of T-cell lineage. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1588–1592. doi: 10.1073/pnas.77.3.1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothlein R., Dustin M. L., Marlin S. D., Springer T. A. A human intercellular adhesion molecule (ICAM-1) distinct from LFA-1. J Immunol. 1986 Aug 15;137(4):1270–1274. [PubMed] [Google Scholar]
- Rothlein R., Springer T. A. The requirement for lymphocyte function-associated antigen 1 in homotypic leukocyte adhesion stimulated by phorbol ester. J Exp Med. 1986 May 1;163(5):1132–1149. doi: 10.1084/jem.163.5.1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Madrid F., Krensky A. M., Ware C. F., Robbins E., Strominger J. L., Burakoff S. J., Springer T. A. Three distinct antigens associated with human T-lymphocyte-mediated cytolysis: LFA-1, LFA-2, and LFA-3. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7489–7493. doi: 10.1073/pnas.79.23.7489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz T. F., Mitterer M., Neumayer H. P., Vogetseder W., Dierich M. P. Involvement in the initiation of T cell responses and structural features of an 85-kDa membrane activation antigen. Eur J Immunol. 1988 Aug;18(8):1253–1258. doi: 10.1002/eji.1830180816. [DOI] [PubMed] [Google Scholar]
- Schulz T. F., Mitterer M., Vogetseder W., Böck G., Myones B. L., Dierich M. P. Identification and characterization of a novel membrane activation antigen with wide cellular distribution. Eur J Immunol. 1988 Jan;18(1):7–11. doi: 10.1002/eji.1830180103. [DOI] [PubMed] [Google Scholar]
- Schulz T. F., Vogetseder W., Mitterer M., Böck G., Johnson J. P., Dierich M. P. Individual epitopes of an 85,000 MW membrane adherence molecule are variably expressed on cells of different lineage. Immunology. 1988 Aug;64(4):581–586. [PMC free article] [PubMed] [Google Scholar]
- Springer T. A., Thompson W. S., Miller L. J., Schmalstieg F. C., Anderson D. C. Inherited deficiency of the Mac-1, LFA-1, p150,95 glycoprotein family and its molecular basis. J Exp Med. 1984 Dec 1;160(6):1901–1918. doi: 10.1084/jem.160.6.1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thaler J., Denz H., Gattringer C., Glassl H., Lechleitner M., Dietze O., Huber H. Diagnostic and prognostic value of immunohistological bone marrow examination: results in 212 patients with lymphoproliferative disorders. Blut. 1987 Apr;54(4):213–222. doi: 10.1007/BF00594196. [DOI] [PubMed] [Google Scholar]
- Vogetseder W., Feichtinger H., Schulz T. F., Schwaeble W., Tabaczewski P., Mitterer M., Böck G., Marth C., Dapunt O., Mikuz G. Expression of 7F7-antigen, a human adhesion molecule identical to intercellular adhesion molecule-1 (ICAM-1) in human carcinomas and their stromal fibroblasts. Int J Cancer. 1989 May 15;43(5):768–773. doi: 10.1002/ijc.2910430504. [DOI] [PubMed] [Google Scholar]



