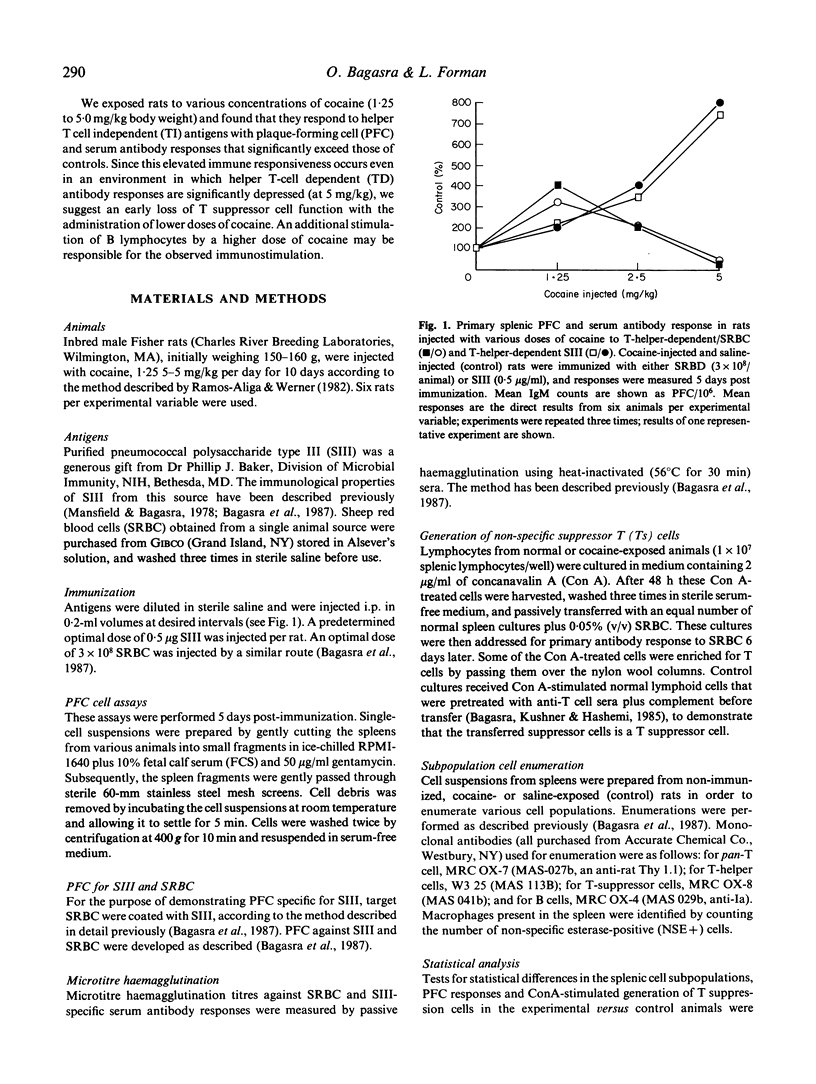
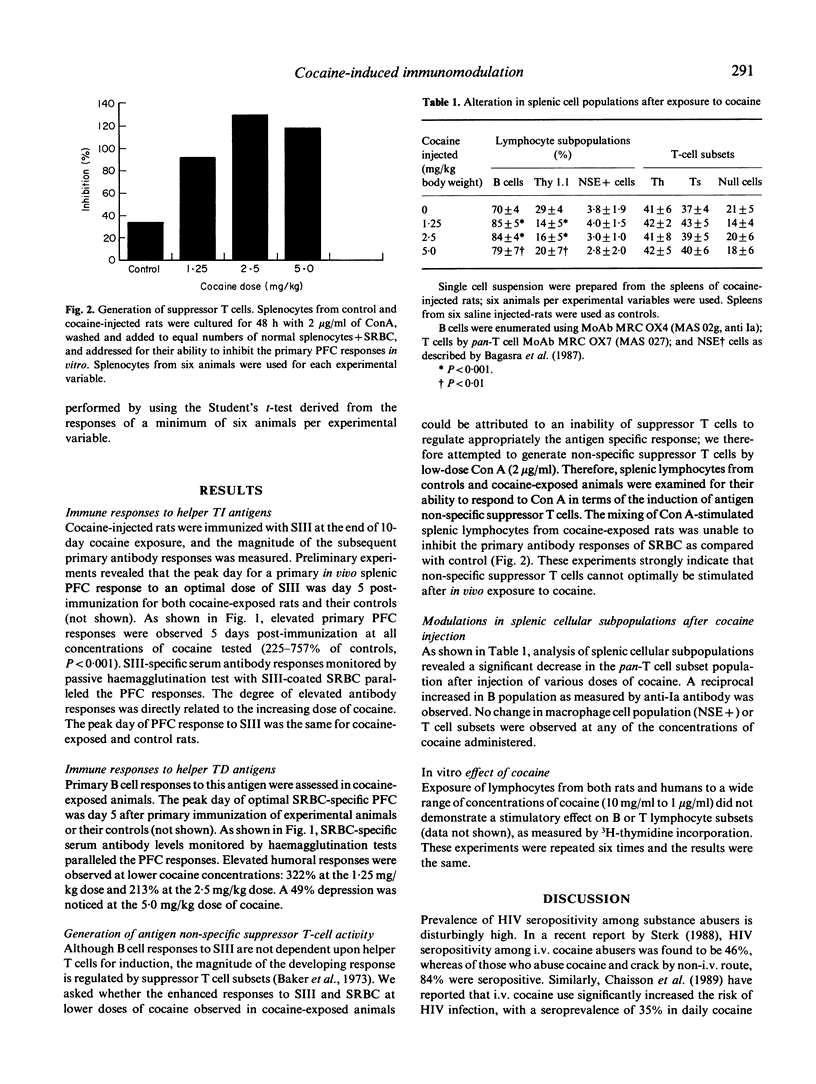
Abstract
The potential role of substance abuse, especially cocaine and alcohol as co-factor in HIV infection and in the development and expression of AIDS has been suggested, but the possible biological role of substance abuse in the development of AIDS is not known. In order to better understand immune system function in chronic cocaine abuse, we have assessed primary B cell responses to helper T-cell independent (TI) and dependent (TD) antigens in inbred Fisher male rats injected with 1.25-5 mg cocaine/kg body weight for 10 days. The ability of cocaine-exposed animals to mount primary in vivo splenic plaque-forming cell (PFC) and serum antibody responses to the TI antigen, pneumococcal polysaccharide type III (SIII), was elevated several-fold when compared with controls. The degree of elevation of humoral antibody responses seemed to be directly related to the dose of cocaine. Primary in vivo B cell responses to the TD antigen, sheep red blood cells (SRBC), was elevated at lower concentrations of cocaine (1.25-2.5 mg/kg) and was found to be significantly suppressed after chronic exposure to the higher concentration (5.0 mg/kg). The elevated primary splenic immunostimulation to TI (SIII) may be attributed to a combination of loss of T suppressor cell control and direct B cell stimulation. Elevated immune responses to SRBC at lower concentrations were attributed to stimulation of T helper cells as well as loss of T suppressor cells. Immunosuppression to SRBC observed in response to 5.0 mg/kg of cocaine was most probably due to loss of T helper cell subset functions. These findings were further tested by in vitro methods, where splenic lymphocytes from cocaine-exposed animals were examined for their ability to respond to concanavalin A (Con A) in terms of the induction of antigen non-specific suppressor T cells. The addition of Con A-stimulated splenic lymphocytes from cocaine-treated animals did not inhibit the primary antibody responses of SRBC as compared with saline-treated controls, indicating that suppressor T cells malfunction after cocaine exposure. Lymphocyte subpopulation analysis using fluorescein-labelled monoclonal antibodies showed a significant increase in the B cell populations at doses of 1.25-5 mg/kg. A reciprocal change in T cell populations also took place. No significant numerical change in macrophage (NSE+) and T cell subset, T helper and T suppressor was noticed, suggesting that cocaine probably directly effects mature T cell subset functions but does not affect their differentiation.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bagasra O., Currao L., DeSouza L. R., Oosterhuis J. W., Damjanov I. Immune response of mice exposed to cis-diamminedichloroplatinum. Cancer Immunol Immunother. 1985;19(2):142–147. doi: 10.1007/BF00199723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagasra O., Howeedy A., Dorio R., Kajdacsy-Balla A. Functional analysis of T-cell subsets in chronic experimental alcoholism. Immunology. 1987 May;61(1):63–69. [PMC free article] [PubMed] [Google Scholar]
- Bagasra O., Kushner H., Hashemi S. Lymphocyte function in experimental endemic syphilis of Syrian hamsters. Immunology. 1985 Sep;56(1):9–21. [PMC free article] [PubMed] [Google Scholar]
- Baker P. J., Reed N. D., Stashak P. W., Amsbaugh D. F., Prescott B. Regulation of the antibody response to type 3 pneumococcal polysaccharide. I. Nature of regulatory cells. J Exp Med. 1973 Jun 1;137(6):1431–1441. doi: 10.1084/jem.137.6.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaisson R. E., Bacchetti P., Osmond D., Brodie B., Sande M. A., Moss A. R. Cocaine use and HIV infection in intravenous drug users in San Francisco. JAMA. 1989 Jan 27;261(4):561–565. [PubMed] [Google Scholar]
- Des Jarlais D. C., Wish E., Friedman S. R., Stoneburner R., Yancovitz S. R., Mildvan D., el-Sadr W., Brady E., Cuadrado M. Intravenous drug use and the heterosexual transmission of the human immunodeficiency virus. Current trends in New York City. N Y State J Med. 1987 May;87(5):283–286. [PubMed] [Google Scholar]
- Forman L. J., Estilow S. Cocaine influences beta-endorphin levels and release. Life Sci. 1988;43(4):309–315. doi: 10.1016/0024-3205(88)90108-7. [DOI] [PubMed] [Google Scholar]
- Friedland G. H., Klein R. S. Transmission of the human immunodeficiency virus. N Engl J Med. 1987 Oct 29;317(18):1125–1135. doi: 10.1056/NEJM198710293171806. [DOI] [PubMed] [Google Scholar]
- Fuchs D., Hausen A., Hoefler E., Schönitzer D., Werner E. R., Dierich M. P., Hengster P., Reibnegger G., Schulz T., Wachter H. Activated T cells in addition to LAV/HTLV-III infection: a necessary precondition for development of AIDS. Cancer Detect Prev Suppl. 1987;1:583–587. [PubMed] [Google Scholar]
- Havas H. F., Dellaria M., Schiffman G., Geller E. B., Adler M. W. Effect of cocaine on the immune response and host resistance in BALB/c mice. Int Arch Allergy Appl Immunol. 1987;83(4):377–383. doi: 10.1159/000234372. [DOI] [PubMed] [Google Scholar]
- Levy N., Carlson J. R., Hinrichs S., Lerche N., Schenker M., Gardner M. B. The prevalence of HTLV-III/LAV antibodies among intravenous drug users attending treatment programs in California: a preliminary report. N Engl J Med. 1986 Feb 13;314(7):446–446. doi: 10.1056/NEJM198602133140711. [DOI] [PubMed] [Google Scholar]
- Mansfield J. M., Bagasra O. Lymphocyte function in experimental African trypanosomiasis. I. B cell responses to helper T cell-independent and -dependent antigens. J Immunol. 1978 Mar;120(3):759–765. [PubMed] [Google Scholar]
- Moss A. R. AIDS and intravenous drug use: the real heterosexual epidemic. Br Med J (Clin Res Ed) 1987 Feb 14;294(6569):389–390. doi: 10.1136/bmj.294.6569.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patarca R., Freeman G. J., Schwartz J., Singh R. P., Kong Q. T., Murphy E., Anderson Y., Sheng F. Y., Singh P., Johnson K. A. rpt-1, an intracellular protein from helper/inducer T cells that regulates gene expression of interleukin 2 receptor and human immunodeficiency virus type 1. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2733–2737. doi: 10.1073/pnas.85.8.2733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn T. C., Glasser D., Cannon R. O., Matuszak D. L., Dunning R. W., Kline R. L., Campbell C. H., Israel E., Fauci A. S., Hook E. W., 3rd Human immunodeficiency virus infection among patients attending clinics for sexually transmitted diseases. N Engl J Med. 1988 Jan 28;318(4):197–203. doi: 10.1056/NEJM198801283180401. [DOI] [PubMed] [Google Scholar]
- Sapolsky R., Rivier C., Yamamoto G., Plotsky P., Vale W. Interleukin-1 stimulates the secretion of hypothalamic corticotropin-releasing factor. Science. 1987 Oct 23;238(4826):522–524. doi: 10.1126/science.2821621. [DOI] [PubMed] [Google Scholar]
- Stall R., McKusick L., Wiley J., Coates T. J., Ostrow D. G. Alcohol and drug use during sexual activity and compliance with safe sex guidelines for AIDS: the AIDS Behavioral Research Project. Health Educ Q. 1986 Winter;13(4):359–371. doi: 10.1177/109019818601300407. [DOI] [PubMed] [Google Scholar]
- Van Dyke C., Stesin A., Jones R., Chuntharapai A., Seaman W. Cocaine increases natural killer cell activity. J Clin Invest. 1986 Apr;77(4):1387–1390. doi: 10.1172/JCI112445. [DOI] [PMC free article] [PubMed] [Google Scholar]



