Abstract
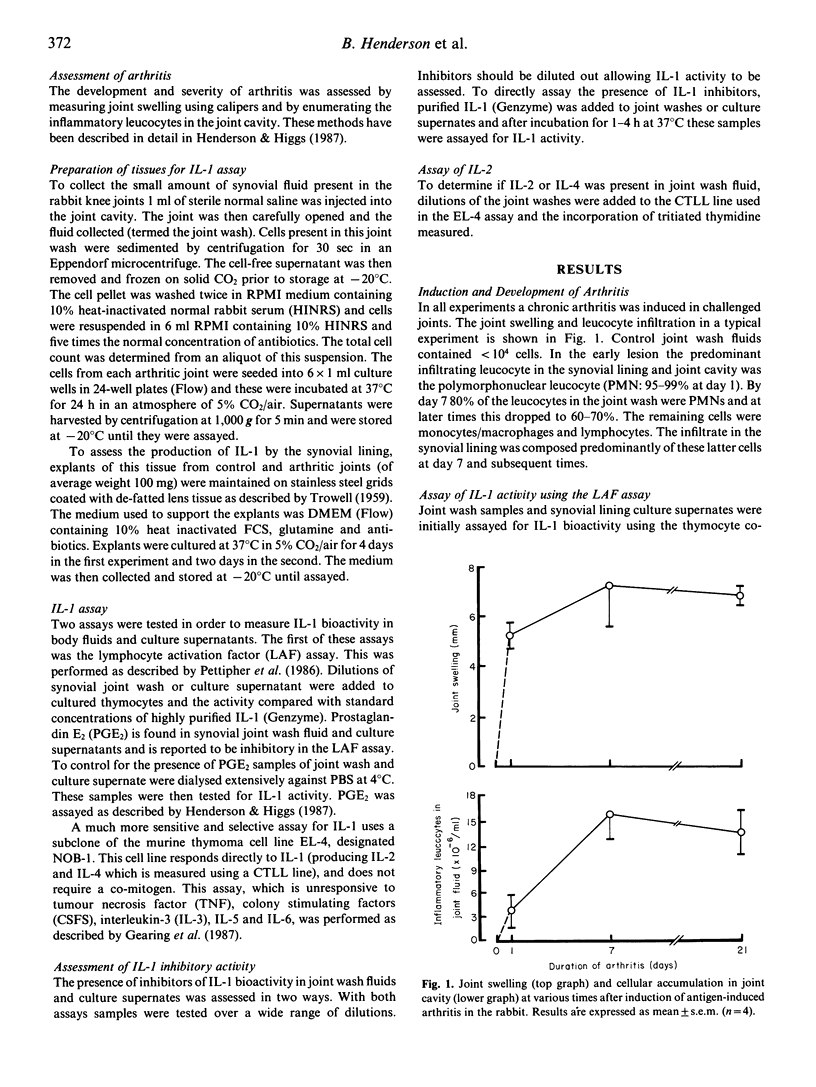
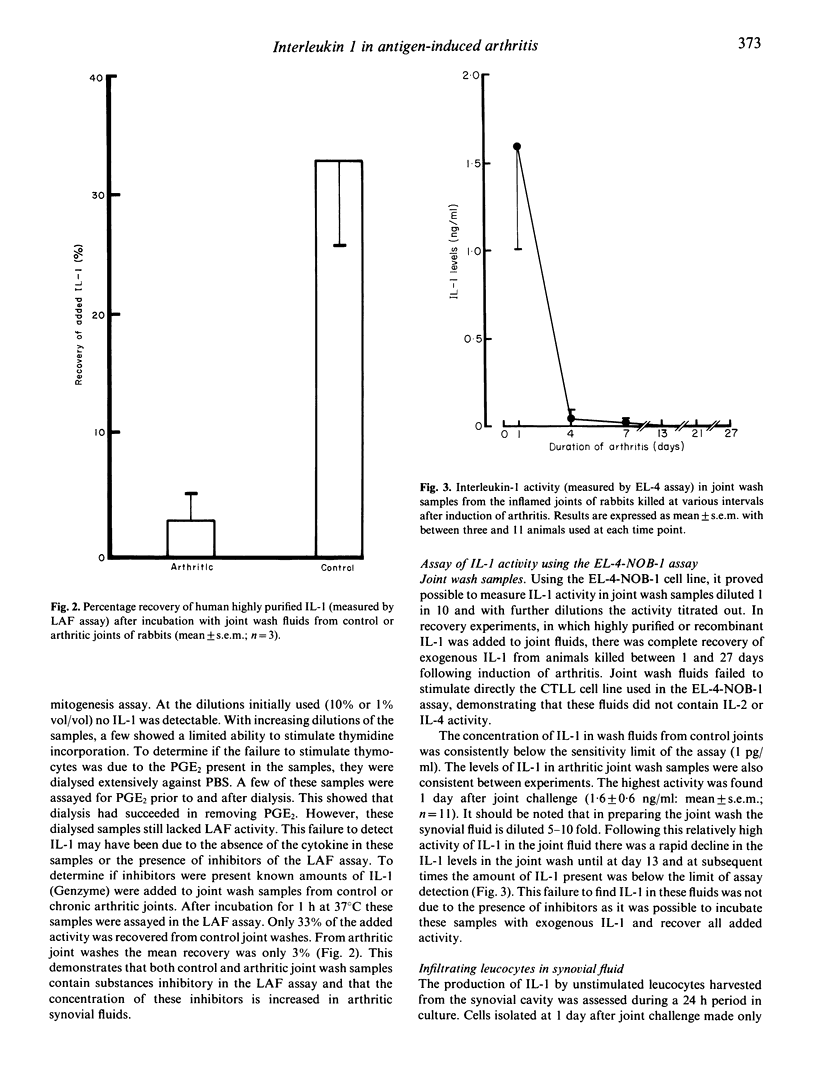
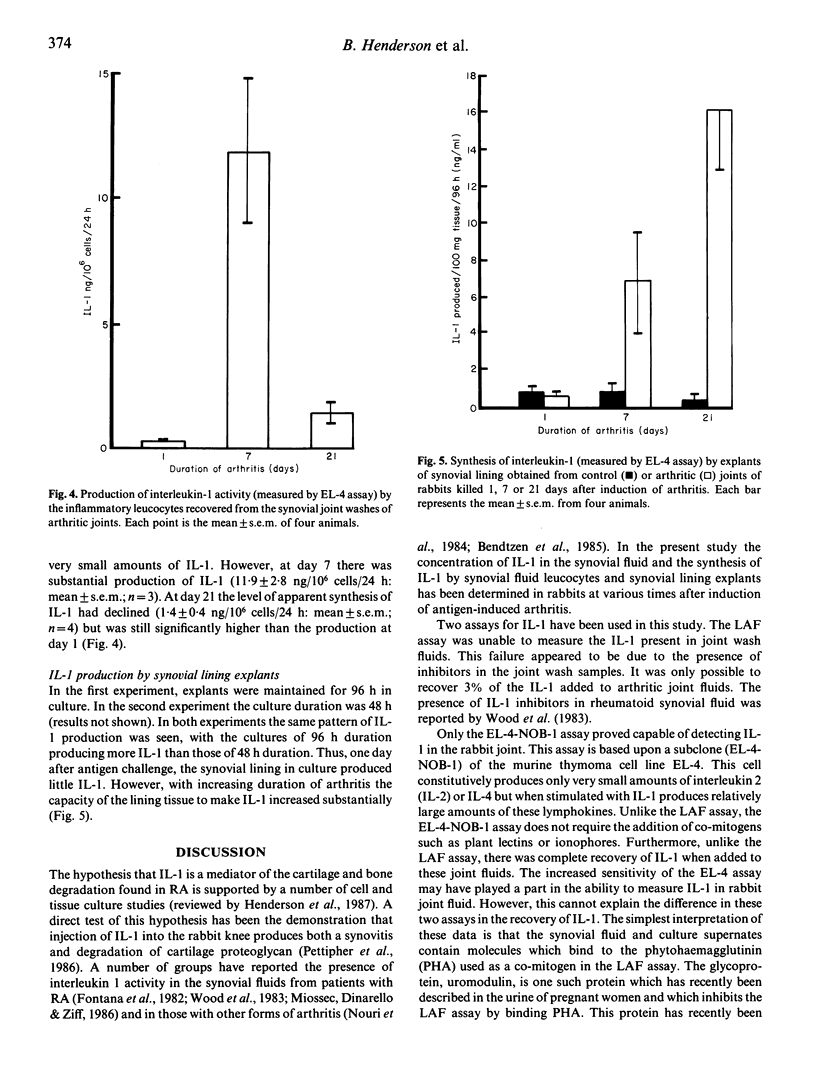
Antigen-induced arthritis in the rabbit closely resembles rheumatoid arthritis. The levels of interleukin 1 (IL-1) in the synovial fluid and the synthesis of IL-1 by infiltrating cells in synovial fluid and by the synovial lining from control and inflamed joints has been assessed during the first month of this disease. A number of biological assays have been used to measure rabbit IL-1. Of these, only the assay using the murine thymoma cell line (EL-4 NOB-1) was able to detect IL-1 activity in the synovial fluid of arthritic joints, which was present only in the very early lesion. The leucocytes infiltrating the synovial cavity produced little IL-1 ex vivo in the acute lesion but released large amounts when arthritis was established. A similar finding was made with respect to the production of IL-1 by the synovial lining.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bendtzen K., Petersen J., Halkjaer-Kristensen J., Ingemann-Hansen T. Interleukin-1-like activities in synovial fluids of patients with rheumatoid arthritis and traumatic synovitis. Rheumatol Int. 1985;5(2):79–82. doi: 10.1007/BF00270301. [DOI] [PubMed] [Google Scholar]
- Consden R., Doble A., Glynn L. E., Nind A. P. Production of a chronic arthritis with ovalbumin. Its retention in the rabbit knee joint. Ann Rheum Dis. 1971 May;30(3):307–315. doi: 10.1136/ard.30.3.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUMONDE D. C., GLYNN L. E. The production of arthritis in rabbits by an immunological reaction to fibrin. Br J Exp Pathol. 1962 Aug;43:373–383. [PMC free article] [PubMed] [Google Scholar]
- Fontana A., Hengartner H., Weber E., Fehr K., Grob P. J., Cohen G. Interleukin 1 activity in the synovial fluid of patients with rheumatoid arthritis. Rheumatol Int. 1982;2(2):49–53. doi: 10.1007/BF00541245. [DOI] [PubMed] [Google Scholar]
- Gearing A. J., Bird C. R., Bristow A., Poole S., Thorpe R. A simple sensitive bioassay for interleukin-1 which is unresponsive to 10(3) U/ml of interleukin-2. J Immunol Methods. 1987 May 4;99(1):7–11. doi: 10.1016/0022-1759(87)90025-1. [DOI] [PubMed] [Google Scholar]
- Henderson B., Glynn L. E. Metabolic alterations in the synoviocytes in chronically inflamed knee joints in immune arthritis in the rabbit: comparison with rheumatoid arthritis. Br J Exp Pathol. 1981 Feb;62(1):27–33. [PMC free article] [PubMed] [Google Scholar]
- Henderson B., Higgs G. A. Synthesis of arachidonate oxidation products by synovial joint tissues during the development of chronic erosive arthritis. Arthritis Rheum. 1987 Oct;30(10):1149–1156. doi: 10.1002/art.1780301010. [DOI] [PubMed] [Google Scholar]
- Henderson B., Pettipher E. R., Higgs G. A. Mediators of rheumatoid arthritis. Br Med Bull. 1987 Apr;43(2):415–428. doi: 10.1093/oxfordjournals.bmb.a072191. [DOI] [PubMed] [Google Scholar]
- Lindemann A., Riedel D., Oster W., Meuer S. C., Blohm D., Mertelsmann R. H., Herrmann F. Granulocyte/macrophage colony-stimulating factor induces interleukin 1 production by human polymorphonuclear neutrophils. J Immunol. 1988 Feb 1;140(3):837–839. [PubMed] [Google Scholar]
- Miossec P., Dinarello C. A., Ziff M. Interleukin-1 lymphocyte chemotactic activity in rheumatoid arthritis synovial fluid. Arthritis Rheum. 1986 Apr;29(4):461–470. doi: 10.1002/art.1780290402. [DOI] [PubMed] [Google Scholar]
- Nouri A. M., Panayi G. S., Goodman S. M. Cytokines and the chronic inflammation of rheumatic disease. I. The presence of interleukin-1 in synovial fluids. Clin Exp Immunol. 1984 Feb;55(2):295–302. [PMC free article] [PubMed] [Google Scholar]
- Pennica D., Kohr W. J., Kuang W. J., Glaister D., Aggarwal B. B., Chen E. Y., Goeddel D. V. Identification of human uromodulin as the Tamm-Horsfall urinary glycoprotein. Science. 1987 Apr 3;236(4797):83–88. doi: 10.1126/science.3453112. [DOI] [PubMed] [Google Scholar]
- Pettipher E. R., Henderson B. The relationship between cell-mediated immunity and cartilage degradation in antigen-induced arthritis in the rabbit. Br J Exp Pathol. 1988 Feb;69(1):113–122. [PMC free article] [PubMed] [Google Scholar]
- Pettipher E. R., Higgs G. A., Henderson B. Interleukin 1 induces leukocyte infiltration and cartilage proteoglycan degradation in the synovial joint. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8749–8753. doi: 10.1073/pnas.83.22.8749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TROWELL O. A. The culture of mature organs in a synthetic medium. Exp Cell Res. 1959 Jan;16(1):118–147. doi: 10.1016/0014-4827(59)90201-0. [DOI] [PubMed] [Google Scholar]
- Wood D. D., Ihrie E. J., Dinarello C. A., Cohen P. L. Isolation of an interleukin-1-like factor from human joint effusions. Arthritis Rheum. 1983 Aug;26(8):975–983. doi: 10.1002/art.1780260806. [DOI] [PubMed] [Google Scholar]



