Abstract
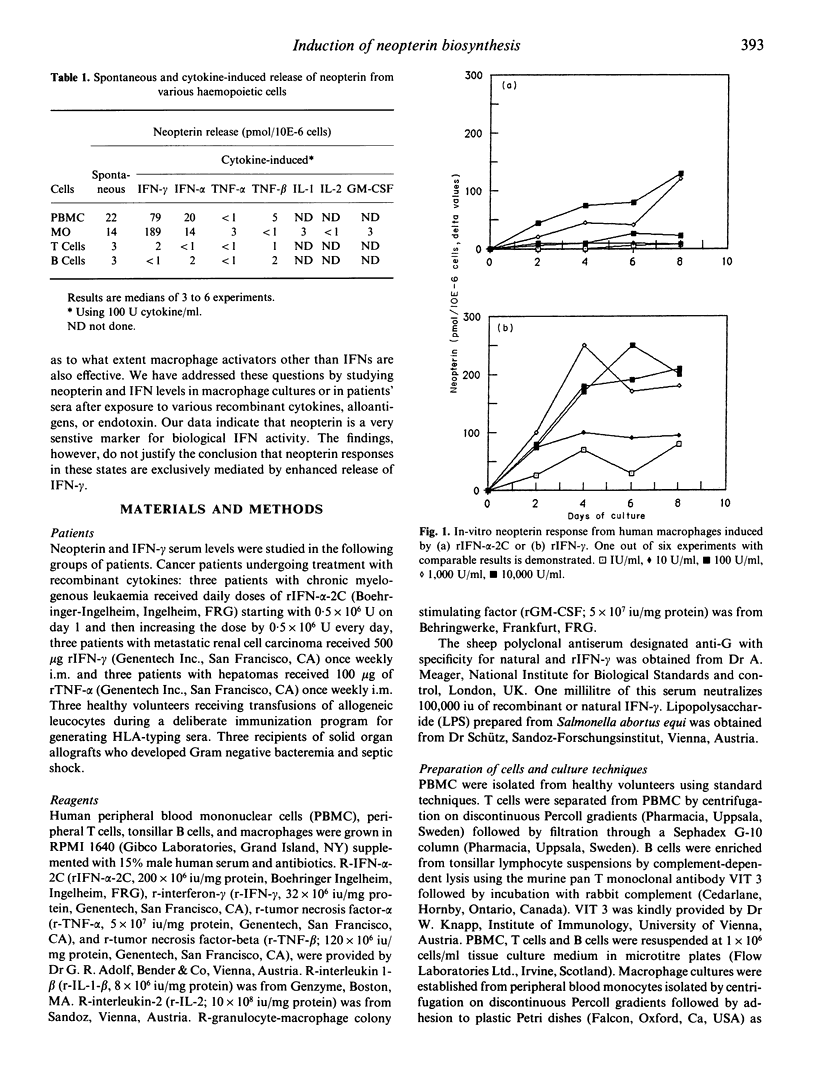
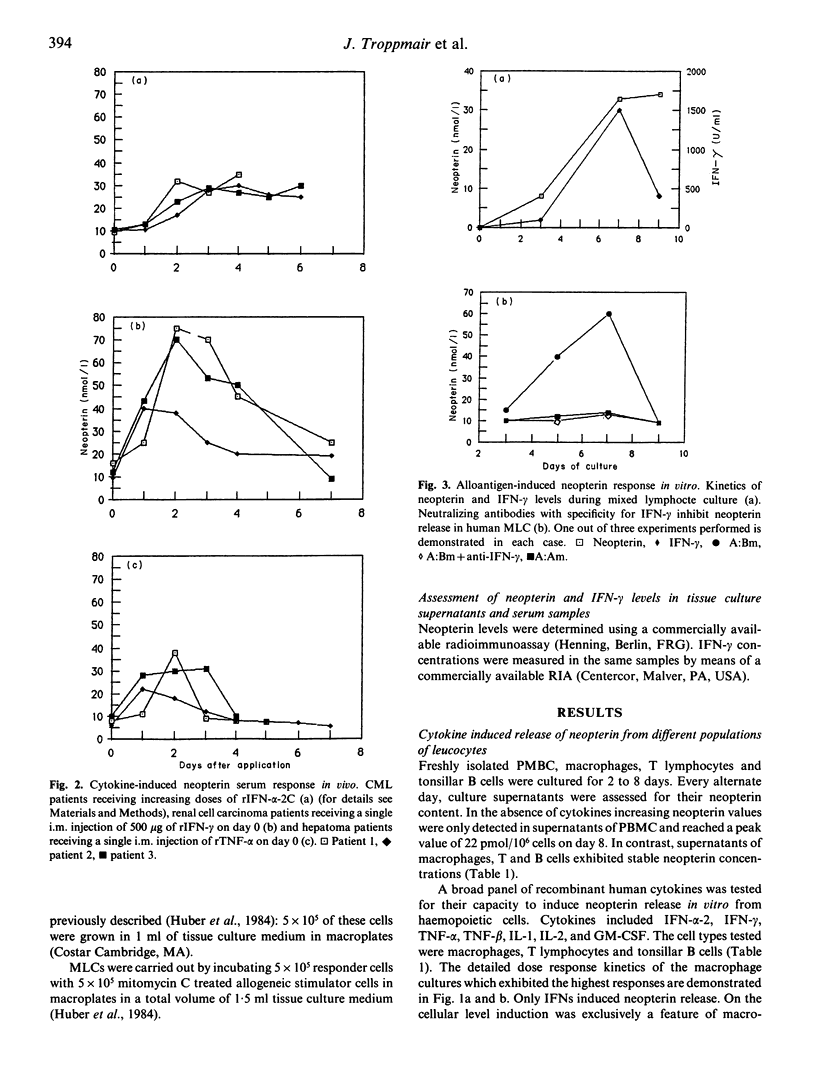
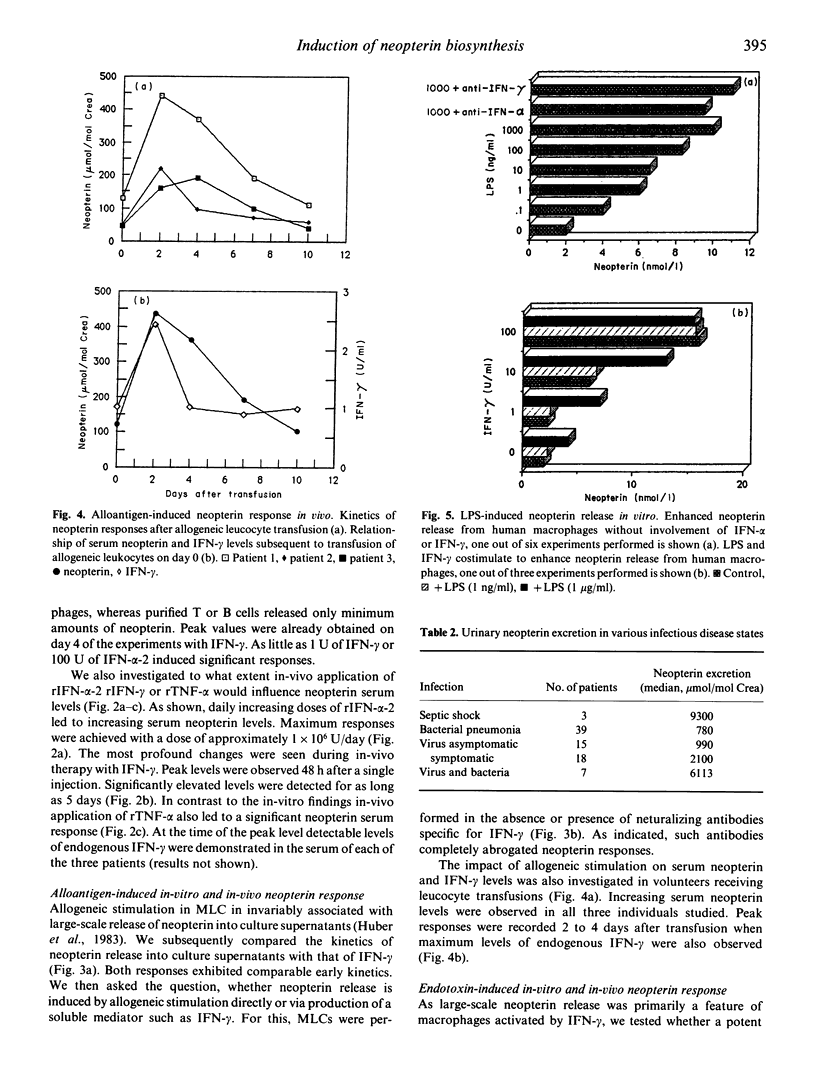
Recently we presented evidence that cellular immune responses are associated with increased in-vitro and in-vivo excretion of neopterin (Huber et al., 1983) and that, in vitro at least, macrophages and IFN-γ play a key role in the induction of this phenomenon (Huber et al., 1984). Although this marker is increasingly applied for monitoring of human disease, there is limited knowledge about the mechanism(s) responsible for its increased biosynthesis during inflammatory states. To further elucidate this question we evaluated neopterin and IFN-levels in culture supernatants of human blood cells and in patients' sera. Cells or patients were exposed to a panel of recombinant cytokines, alloantigens or lipopolysaccharide. To investigate indirect stimulation by induction of production of endogenous IFNs, the impact of neutralization of IFNs by addition of specific antibodies was also studied. The data confirm our previous results which identified the monocyte/macrophage as the main producer cell among human blood cells. They further demonstrate that, at least in vitro, IFN-γ, IFN-α and LPS can all stimulate neopterin release independently from each other. Thirdly, they indicate that stimuli such as alloantigens or TNF-α can indirectly enhance neopterin release by their capacity to induce production of endogenous IFN-γ. On the basis of these data we conclude that enhanced neopterin biosynthesis does not necessarily relate to activation of T cells but can also be caused by non-immune stimuli.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abb J., Abb H., Deinhardt F. Phenotype of human alpha-interferon producing leucocytes identified by monoclonal antibodies. Clin Exp Immunol. 1983 Apr;52(1):179–184. [PMC free article] [PubMed] [Google Scholar]
- Bancroft G. J., Schreiber R. D., Bosma G. C., Bosma M. J., Unanue E. R. A T cell-independent mechanism of macrophage activation by interferon-gamma. J Immunol. 1987 Aug 15;139(4):1104–1107. [PubMed] [Google Scholar]
- Fuchs D., Hausen A., Kofler M., Kosanowski H., Reibnegger G., Wachter H. Neopterin as an index of immune response in patients with tuberculosis. Lung. 1984;162(6):337–346. doi: 10.1007/BF02715666. [DOI] [PubMed] [Google Scholar]
- Huber C., Batchelor J. R., Fuchs D., Hausen A., Lang A., Niederwieser D., Reibnegger G., Swetly P., Troppmair J., Wachter H. Immune response-associated production of neopterin. Release from macrophages primarily under control of interferon-gamma. J Exp Med. 1984 Jul 1;160(1):310–316. doi: 10.1084/jem.160.1.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber C., Fuchs D., Hausen A., Margreiter R., Reibnegger G., Spielberger M., Wachter H. Pteridines as a new marker to detect human T cells activated by allogeneic or modified self major histocompatibility complex (MHC) determinants. J Immunol. 1983 Mar;130(3):1047–1050. [PubMed] [Google Scholar]
- Kasahara T., Hooks J. J., Dougherty S. F., Oppenheim J. J. Interleukin 2-mediated immune interferon (IFN-gamma) production by human T cells and T cell subsets. J Immunol. 1983 Apr;130(4):1784–1789. [PubMed] [Google Scholar]
- Niederwieser A., Curtius H. C., Bettoni O., Bieri J., Schircks B., Viscontini M., Schaub J. Atypical phenylketonuria caused by 7, 8-dihydrobiopterin synthetase deficiency. Lancet. 1979 Jan 20;1(8108):131–133. doi: 10.1016/s0140-6736(79)90521-x. [DOI] [PubMed] [Google Scholar]
- Niederwieser D., Huber C., Gratwohl A., Bannert P., Fuchs D., Hausen A., Reibnegger G., Speck B., Wachter H. Neopterin as a new biochemical marker in the clinical monitoring of bone marrow transplant recipients. Transplantation. 1984 Nov;38(5):497–500. doi: 10.1097/00007890-198411000-00011. [DOI] [PubMed] [Google Scholar]
- Schoedon G., Troppmair J., Adolf G., Huber C., Niederwieser A. Interferon-gamma enhances biosynthesis of pterins in peripheral blood mononuclear cells by induction of GTP-cyclohydrolase I activity. J Interferon Res. 1986 Dec;6(6):697–703. doi: 10.1089/jir.1986.6.697. [DOI] [PubMed] [Google Scholar]
- Schoedon G., Troppmair J., Fontana A., Huber C., Curtius H. C., Niederwieser A. Biosynthesis and metabolism of pterins in peripheral blood mononuclear cells and leukemia lines of man and mouse. Eur J Biochem. 1987 Jul 15;166(2):303–310. doi: 10.1111/j.1432-1033.1987.tb13515.x. [DOI] [PubMed] [Google Scholar]
- Wachter H., Fuchs D., Hausen A., Reibnegger G., Werner E. R., Dierich M. P. Who will get AIDS? Lancet. 1986 Nov 22;2(8517):1216–1217. doi: 10.1016/s0140-6736(86)92220-8. [DOI] [PubMed] [Google Scholar]
- Wachter H., Hausen A., Grassmayr K. Erhöhte Ausscheidung von Neopterin im Harn von Patienten mit malignen Tumoren und mit Viruserkrankungen. Hoppe Seylers Z Physiol Chem. 1979 Dec;360(12):1957–1960. [PubMed] [Google Scholar]



