Abstract
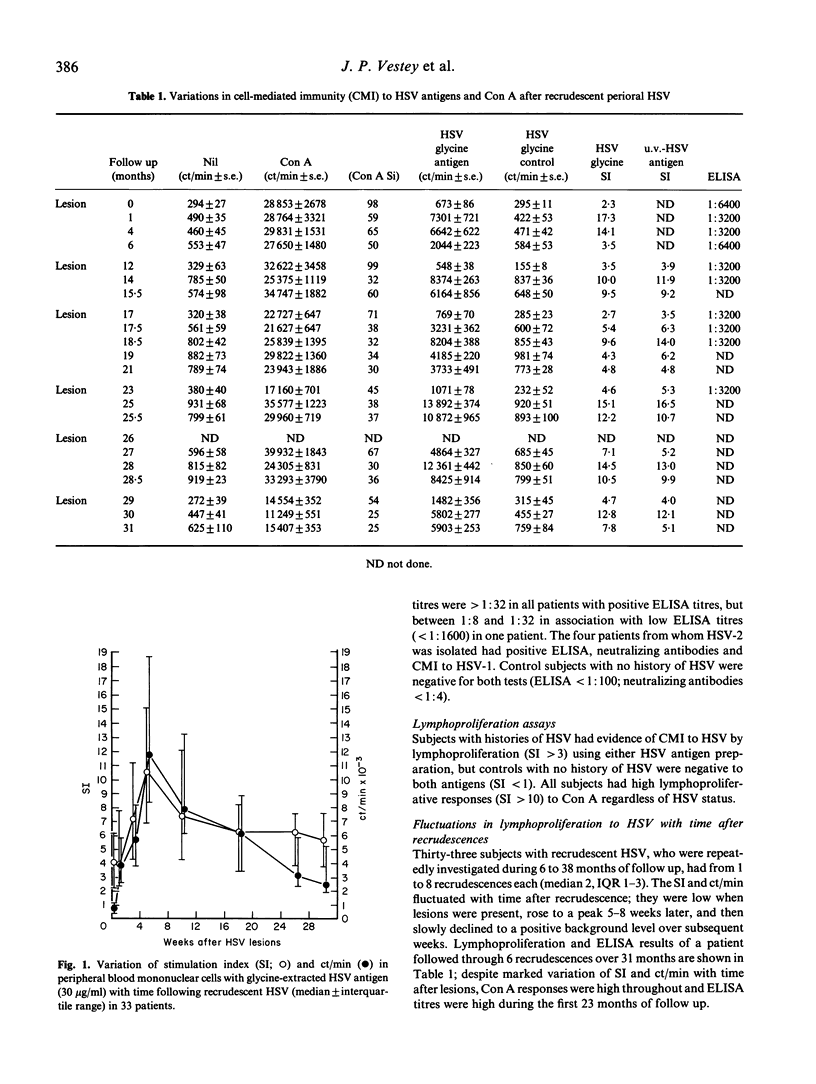
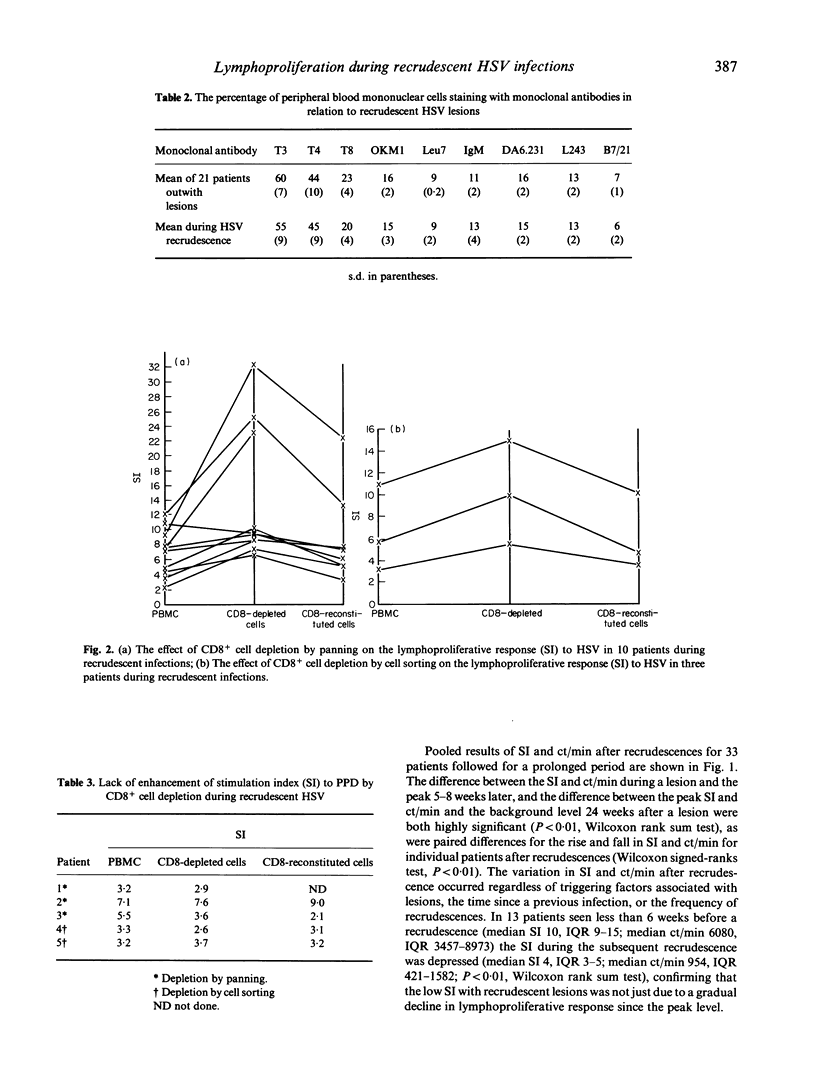
Sixty-five patients with recrudescent orofacial herpes simplex virus (HSV) infections all had circulating HSV-specific antibody measured by ELISA and cell-mediated immunity (CMI) to HSV by in vitro lymphoproliferation. Thirteen control subjects with no history of HSV were negative for both tests. Thirty-three patients, repeatedly investigated during 6 to 38 months, had between 1 and 8 recrudescences each. Lymphoproliferative responses to HSV were low during recrudescence, rose to a peak a few weeks later and then declined to a positive background level. However, ELISA titres and lymphoproliferative responses to concanavalin A were high throughout, and peripheral blood mononuclear cell (PBMC) subset numbers measured by fluorescent flow cytometry remained within normal limits. During HSV lesions, depressed lymphoproliferation to HSV was abrogated by removal of CD8+ T cells from PBMC either by using a panning technique (nine patients) or by cell sorting (three patients). Reconstitution of the CD8-depleted population suppressed the lymphoproliferative response to HSV. Depletion of CD8+ T cells did not affect lymphoproliferation to HSV outwith recrudescence (four patients), nor lymphoproliferative responses to another antigen (PPD; five patients) during recrudescence. Thus, reduced lymphoproliferation to HSV during recrudescence may be due to HSV-specific CD8+ suppressor T lymphocyte function, rather than lack of HSV-responsive lymphocytes. This may result in depression of normal CMI responses to the virus during an asymptomatic recurrence allowing recrudescent lesions to develop.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDERSON W. A., KILBOURNE E. D. A herpes simplex skin test diagnostic antigen of low protein content from cell culture fluid. J Invest Dermatol. 1961 Jul;37:25–28. [PubMed] [Google Scholar]
- Blacklaws B. A., Nash A. A., Darby G. Specificity of the immune response of mice to herpes simplex virus glycoproteins B and D constitutively expressed on L cell lines. J Gen Virol. 1987 Apr;68(Pt 4):1103–1114. doi: 10.1099/0022-1317-68-4-1103. [DOI] [PubMed] [Google Scholar]
- Corey L., Reeves W. C., Holmes K. K. Cellular immune response in genital herpes simplex virus infection. N Engl J Med. 1978 Nov 2;299(18):986–991. doi: 10.1056/NEJM197811022991805. [DOI] [PubMed] [Google Scholar]
- Corey L., Spear P. G. Infections with herpes simplex viruses (1). N Engl J Med. 1986 Mar 13;314(11):686–691. doi: 10.1056/NEJM198603133141105. [DOI] [PubMed] [Google Scholar]
- El Araby I. I., Chernesky M. A., Rawls W. E., Dent P. B. Depressed herpes simplex virus-induced lymphocyte blastogenesis in individuals with severe recurrent herpes infections. Clin Immunol Immunopathol. 1978 Feb;9(2):253–263. doi: 10.1016/0090-1229(78)90078-8. [DOI] [PubMed] [Google Scholar]
- Glorioso J., Kees U., Kümel G., Kirchner H., Krammer P. H. Identification of herpes simplex virus type 1 (HSV-1) glycoprotein gC as the immunodominant antigen for HSV-1-specific memory cytotoxic T lymphocytes. J Immunol. 1985 Jul;135(1):575–582. [PubMed] [Google Scholar]
- Heber-Katz E., Valentine S., Dietzschold B., Burns-Purzycki C. Overlapping T cell antigenic sites on a synthetic peptide fragment from herpes simplex virus glycoprotein D, the degenerate MHC restriction elicited, and functional evidence for antigen-Ia interaction. J Exp Med. 1988 Feb 1;167(2):275–287. doi: 10.1084/jem.167.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howie S. E., Ross J. A., Norval M., Maingay J. P. In vivo modulation of antigen presentation generates Ts rather than TDH in HSV-1 infection. Immunology. 1987 Mar;60(3):419–423. [PMC free article] [PubMed] [Google Scholar]
- Iwasaka T., Sheridan J. F., Aurelian L. Immunity to herpes simplex virus type 2: recurrent lesions are associated with the induction of suppressor cells and soluble suppressor factors. Infect Immun. 1983 Dec;42(3):955–964. doi: 10.1128/iai.42.3.955-964.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalimo K. O., Joronen I. A., Havu V. K. Cell-mediated immunity against herpes simplex virus envelope, capsid, excreted, and crude antigens. Infect Immun. 1983 Jan;39(1):24–28. doi: 10.1128/iai.39.1.24-28.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez C., O'Reilly R. J. Cell-mediated immune responses in recurrent herpesvirus infections. I. Lymphocyte proliferation assay. J Immunol. 1977 Mar;118(3):895–902. [PubMed] [Google Scholar]
- Martin S., Courtney R. J., Fowler G., Rouse B. T. Herpes simplex virus type 1-specific cytotoxic T lymphocytes recognize virus nonstructural proteins. J Virol. 1988 Jul;62(7):2265–2273. doi: 10.1128/jvi.62.7.2265-2273.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen L., Merigan T. C. Role of T lymphocytes in cellular immune responses during herpes simplex virus infection in humans. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3957–3961. doi: 10.1073/pnas.75.8.3957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rattray M. C., Peterman G. M., Altman L. C., Corey L., Holmes K. K. Lymphocyte-derived chemotactic factor synthesis in initial genital herpesvirus infection: correlation with lymphocyte transformation. Infect Immun. 1980 Oct;30(1):110–116. doi: 10.1128/iai.30.1.110-116.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouse B. T., Horohov D. W. Immunosuppression in viral infections. Rev Infect Dis. 1986 Nov-Dec;8(6):850–873. doi: 10.1093/clinids/8.6.850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauder D. N., Noonan F. P., DeFabo E. C., Katz S. I. Ultraviolet radiation inhibits alloantigen presentation by epidermal cells: partial reversal by the soluble epidermal cell product, epidermal cell-derived thymocyte-activating factor (ETAF). J Invest Dermatol. 1983 Jun;80(6):485–489. doi: 10.1111/1523-1747.ep12534951. [DOI] [PubMed] [Google Scholar]
- Sheridan J. F., Beck M., Aurelian L., Radowsky M. Immunity to herpes simplex virus: virus reactivation modulates lymphokine activity. J Infect Dis. 1985 Sep;152(3):449–456. doi: 10.1093/infdis/152.3.449. [DOI] [PubMed] [Google Scholar]
- Sheridan J. F., Beck M., Smith C. C., Aurelian L. Reactivation of herpes simplex virus is associated with production of a low molecular weight factor that inhibits lymphokine activity in vitro. J Immunol. 1987 Feb 15;138(4):1234–1239. [PubMed] [Google Scholar]
- Sheridan J. F., Donnenberg A. D., Aurelian L., Elpern D. J. Immunity to herpes simplex virus type 2. IV. Impaired lymphokine production during recrudescence correlates with an imbalance in T lymphocyte subsets. J Immunol. 1982 Jul;129(1):326–331. [PubMed] [Google Scholar]
- Shillitoe E. J., Wilton J. M., Lehner T. Sequential changes in T and B lymphocyte responses to Herpes simplex virus in man. Scand J Immunol. 1978;7(5):357–366. doi: 10.1111/j.1365-3083.1978.tb00465.x. [DOI] [PubMed] [Google Scholar]
- Shillitoe E. J., Wilton J. M., Lehner T. Sequential changes in cell-mediated immune responses to herpes simplex virus after recurrent herpetic infection in humans. Infect Immun. 1977 Oct;18(1):130–137. doi: 10.1128/iai.18.1.130-137.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens J. G., Haarr L., Porter D. D., Cook M. L., Wagner E. K. Prominence of the herpes simplex virus latency-associated transcript in trigeminal ganglia from seropositive humans. J Infect Dis. 1988 Jul;158(1):117–123. doi: 10.1093/infdis/158.1.117. [DOI] [PubMed] [Google Scholar]
- Tsutsumi H., Bernstein J. M., Riepenhoff-Talty M., Cohen E., Orsini F., Ogra P. L. Immune responses to herpes simplex virus in patients with recurrent herpes labialis: I. Development of cell-mediated cytotoxic responses. Clin Exp Immunol. 1986 Dec;66(3):507–515. [PMC free article] [PubMed] [Google Scholar]
- Vestey J. P., Howie S. E., Norval M., Maingay J. P., Neill W. A. Immune responses to herpes simplex virus in patients with facial herpes simplex and those with eczema herpeticum. Br J Dermatol. 1988 Jun;118(6):775–782. doi: 10.1111/j.1365-2133.1988.tb02595.x. [DOI] [PubMed] [Google Scholar]
- Wainberg M. A., Portnoy J. D., Clecner B., Hubschman S., Lagacé-Simard J., Rabinovitch N., Remer Z., Mendelson J. Viral inhibition of lymphocyte proliferative responsiveness in patients suffering from recurrent lesions caused by herpes simplex virus. J Infect Dis. 1985 Sep;152(3):441–448. doi: 10.1093/infdis/152.3.441. [DOI] [PubMed] [Google Scholar]
- Wildy P., Gell P. G. The host response to herpes simplex virus. Br Med Bull. 1985 Jan;41(1):86–91. doi: 10.1093/oxfordjournals.bmb.a072032. [DOI] [PubMed] [Google Scholar]
- Zweerink H. J., Stanton L. W. Immune response to herpes simplex virus infections: virus-specific antibodies in sera from patients with recurrent facial infections. Infect Immun. 1981 Feb;31(2):624–630. doi: 10.1128/iai.31.2.624-630.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]



