Abstract
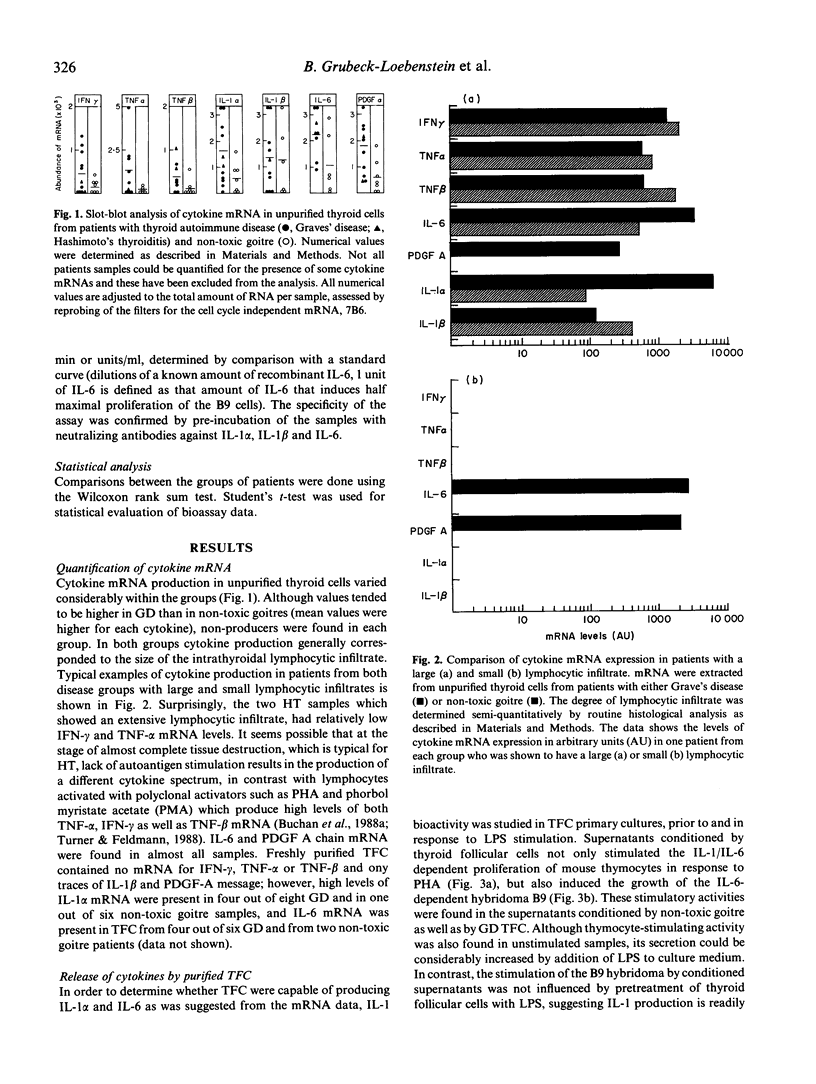
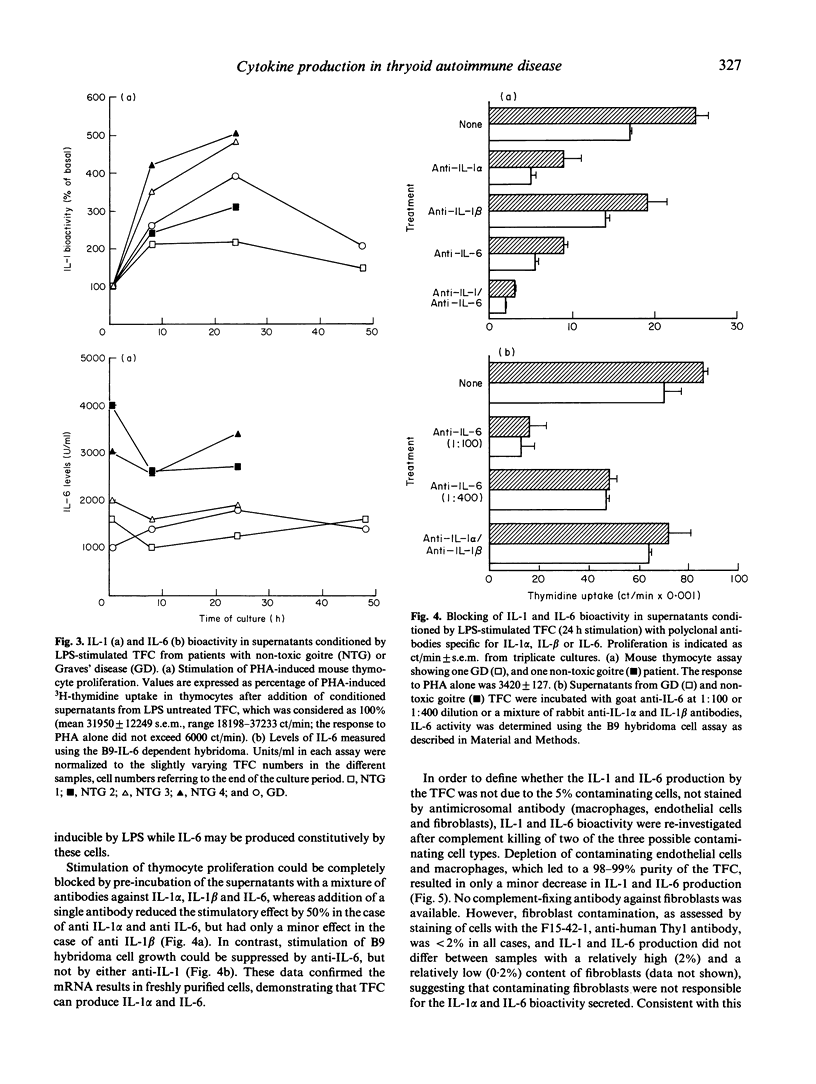
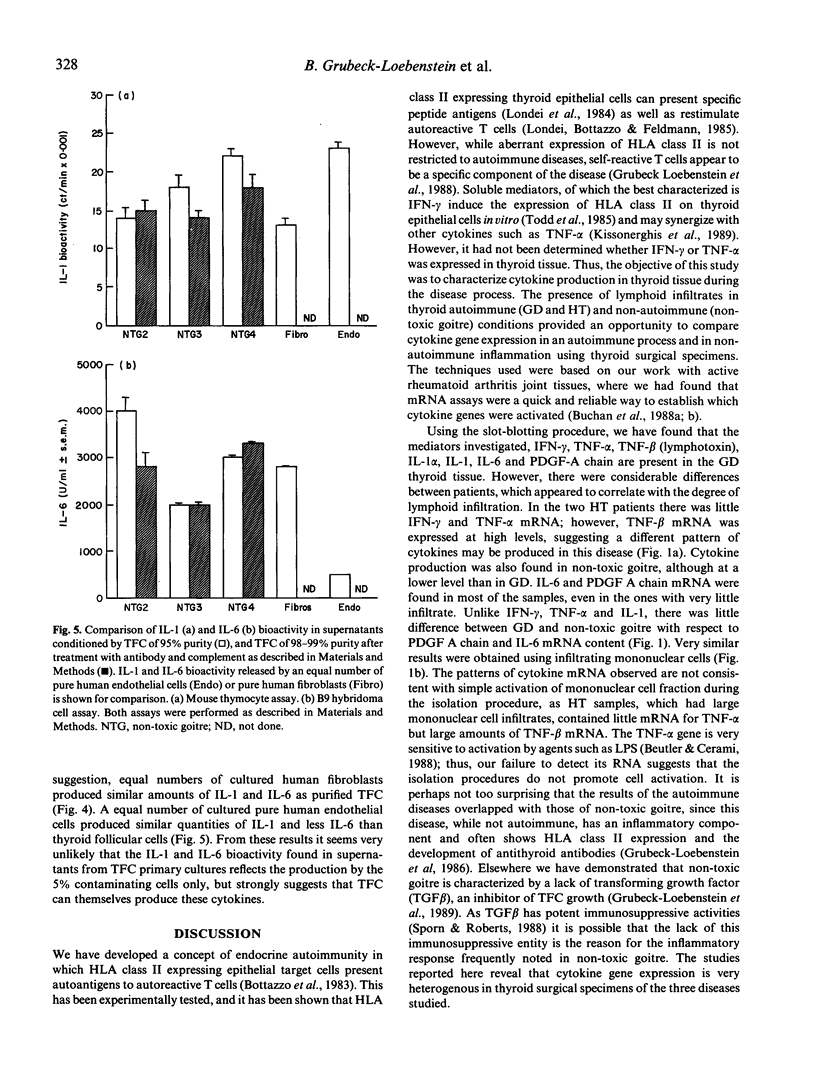
Cytokine production was studied in thyroid tissue from patients with Graves' disease, Hashimoto's thyroiditis and non-toxic goitre. The expression of interferon gamma, tumour necrosis factor alpha and beta, interleukin-1 alpha and beta, interleukin-6 and platelet-derived growth factor A chain was assessed by slot-blot analysis of the respective mRNA in freshly isolated tissue samples. All seven cytokines were detected in patients of all groups. Although the respective mRNA levels were, in general, higher in thyroid autoimmune disorders, this appeared to relate to the degree of the lymphocytic infiltration of the thyroid gland at the time of surgery. Purified thyroid follicular cells expressed high levels of interleukin-1 alpha and interleukin-6 mRNA and when established in primary culture, purified thyroid follicular cells from Graves' disease as well as non-toxic goitre produced interleukin-1 alpha and interleukin-6 bioactivity spontaneously. In the case of interleukin-1 this could be further augmented by addition of lipopolysaccharide to the thyroid follicular cell cultures. These results demonstrate that the lymphocytic infiltrate found in autoimmune and non-autoimmune thyroid disorders is associated with cytokine production. Additionally we have shown that intrathyroidal cytokine production is not restricted to thyroid-infiltrating mononuclear cells, but may also involve thyroid follicular cells both in vivo and in vitro. The cytokines produced by thyroid follicular cells may have an important role in stimulating autoantigen specific T cells in vivo as both interleukin-1 and interleukin-6 facilitate T cell activation.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aarden L. A., De Groot E. R., Schaap O. L., Lansdorp P. M. Production of hybridoma growth factor by human monocytes. Eur J Immunol. 1987 Oct;17(10):1411–1416. doi: 10.1002/eji.1830171004. [DOI] [PubMed] [Google Scholar]
- Baroja M. L., Ceuppens J. L., Van Damme J., Billiau A. Cooperation between an anti-T cell (anti-CD28) monoclonal antibody and monocyte-produced IL-6 in the induction of T cell responsiveness to IL-2. J Immunol. 1988 Sep 1;141(5):1502–1507. [PubMed] [Google Scholar]
- Betsholtz C., Johnsson A., Heldin C. H., Westermark B., Lind P., Urdea M. S., Eddy R., Shows T. B., Philpott K., Mellor A. L. cDNA sequence and chromosomal localization of human platelet-derived growth factor A-chain and its expression in tumour cell lines. Nature. 1986 Apr 24;320(6064):695–699. doi: 10.1038/320695a0. [DOI] [PubMed] [Google Scholar]
- Beutler B., Cerami A. The history, properties, and biological effects of cachectin. Biochemistry. 1988 Oct 4;27(20):7575–7582. doi: 10.1021/bi00420a001. [DOI] [PubMed] [Google Scholar]
- Bottazzo G. F., Pujol-Borrell R., Hanafusa T., Feldmann M. Role of aberrant HLA-DR expression and antigen presentation in induction of endocrine autoimmunity. Lancet. 1983 Nov 12;2(8359):1115–1119. doi: 10.1016/s0140-6736(83)90629-3. [DOI] [PubMed] [Google Scholar]
- Buchan G., Barrett K., Fujita T., Taniguchi T., Maini R., Feldmann M. Detection of activated T cell products in the rheumatoid joint using cDNA probes to Interleukin-2 (IL-2) IL-2 receptor and IFN-gamma. Clin Exp Immunol. 1988 Feb;71(2):295–301. [PMC free article] [PubMed] [Google Scholar]
- Buchan G., Barrett K., Turner M., Chantry D., Maini R. N., Feldmann M. Interleukin-1 and tumour necrosis factor mRNA expression in rheumatoid arthritis: prolonged production of IL-1 alpha. Clin Exp Immunol. 1988 Sep;73(3):449–455. [PMC free article] [PubMed] [Google Scholar]
- Grubeck-Loebenstein B., Buchan G., Sadeghi R., Kissonerghis M., Londei M., Turner M., Pirich K., Roka R., Niederle B., Kassal H. Transforming growth factor beta regulates thyroid growth. Role in the pathogenesis of nontoxic goiter. J Clin Invest. 1989 Mar;83(3):764–770. doi: 10.1172/JCI113955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grubeck-Loebenstein B., Kassal H., Smyth P. P., Krisch K., Waldhäusl W. The prevalence of immunological abnormalities in endemic simple goitre. Acta Endocrinol (Copenh) 1986 Dec;113(4):508–513. doi: 10.1530/acta.0.1130508. [DOI] [PubMed] [Google Scholar]
- Grubeck-Loebenstein B., Londei M., Greenall C., Pirich K., Kassal H., Waldhäusl W., Feldmann M. Pathogenetic relevance of HLA class II expressing thyroid follicular cells in nontoxic Goiter and in Graves' disease. J Clin Invest. 1988 May;81(5):1608–1614. doi: 10.1172/JCI113495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano T., Yasukawa K., Harada H., Taga T., Watanabe Y., Matsuda T., Kashiwamura S., Nakajima K., Koyama K., Iwamatsu A. Complementary DNA for a novel human interleukin (BSF-2) that induces B lymphocytes to produce immunoglobulin. Nature. 1986 Nov 6;324(6092):73–76. doi: 10.1038/324073a0. [DOI] [PubMed] [Google Scholar]
- Hirose W., Kawagoe M., Hara M., Kitani A., Hirose T., Norioka K., Harigai M., Nakamura H. Production of thymocyte-stimulating activity by cultured human thyroid epithelial cells. Clin Exp Immunol. 1987 Oct;70(1):102–109. [PMC free article] [PubMed] [Google Scholar]
- Houssiau F. A., Coulie P. G., Olive D., Van Snick J. Synergistic activation of human T cells by interleukin 1 and interleukin 6. Eur J Immunol. 1988 Apr;18(4):653–656. doi: 10.1002/eji.1830180427. [DOI] [PubMed] [Google Scholar]
- Kaczmarek L., Calabretta B., Baserga R. Expression of cell-cycle-dependent genes in phytohemagglutinin-stimulated human lymphocytes. Proc Natl Acad Sci U S A. 1985 Aug;82(16):5375–5379. doi: 10.1073/pnas.82.16.5375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd R. V., Johnson T. L., Blaivas M., Sisson J. C., Wilson B. S. Detection of HLA-DR antigens in paraffin-embedded thyroid epithelial cells with a monoclonal antibody. Am J Pathol. 1985 Jul;120(1):106–111. [PMC free article] [PubMed] [Google Scholar]
- Londei M., Bottazzo G. F., Feldmann M. Human T-cell clones from autoimmune thyroid glands: specific recognition of autologous thyroid cells. Science. 1985 Apr 5;228(4695):85–89. doi: 10.1126/science.3871967. [DOI] [PubMed] [Google Scholar]
- Londei M., Lamb J. R., Bottazzo G. F., Feldmann M. Epithelial cells expressing aberrant MHC class II determinants can present antigen to cloned human T cells. Nature. 1984 Dec 13;312(5995):639–641. doi: 10.1038/312639a0. [DOI] [PubMed] [Google Scholar]
- Lucas-Martin A., Foz-Sala M., Todd I., Bottazzo G. F., Pujol-Borrell R. Occurrence of thyrocyte HLA class II expression in a wide variety of thyroid diseases: relationship with lymphocytic infiltration and thyroid autoantibodies. J Clin Endocrinol Metab. 1988 Feb;66(2):367–375. doi: 10.1210/jcem-66-2-367. [DOI] [PubMed] [Google Scholar]
- Sporn M. B., Roberts A. B. Peptide growth factors are multifunctional. Nature. 1988 Mar 17;332(6161):217–219. doi: 10.1038/332217a0. [DOI] [PubMed] [Google Scholar]
- Taga T., Kawanishi Y., Hardy R. R., Hirano T., Kishimoto T. Receptors for B cell stimulatory factor 2. Quantitation, specificity, distribution, and regulation of their expression. J Exp Med. 1987 Oct 1;166(4):967–981. doi: 10.1084/jem.166.4.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd I., Pujol-Borrell R., Hammond L. J., Bottazzo G. F., Feldmann M. Interferon-gamma induces HLA-DR expression by thyroid epithelium. Clin Exp Immunol. 1985 Aug;61(2):265–273. [PMC free article] [PubMed] [Google Scholar]
- Turner M., Feldmann M. Comparison of patterns of expression of tumour necrosis factor, lymphotoxin and interleukin-6 mRNA. Biochem Biophys Res Commun. 1988 Jun 30;153(3):1144–1151. doi: 10.1016/s0006-291x(88)81347-0. [DOI] [PubMed] [Google Scholar]
- Uyttenhove C., Coulie P. G., Van Snick J. T cell growth and differentiation induced by interleukin-HP1/IL-6, the murine hybridoma/plasmacytoma growth factor. J Exp Med. 1988 Apr 1;167(4):1417–1427. doi: 10.1084/jem.167.4.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]



