Abstract
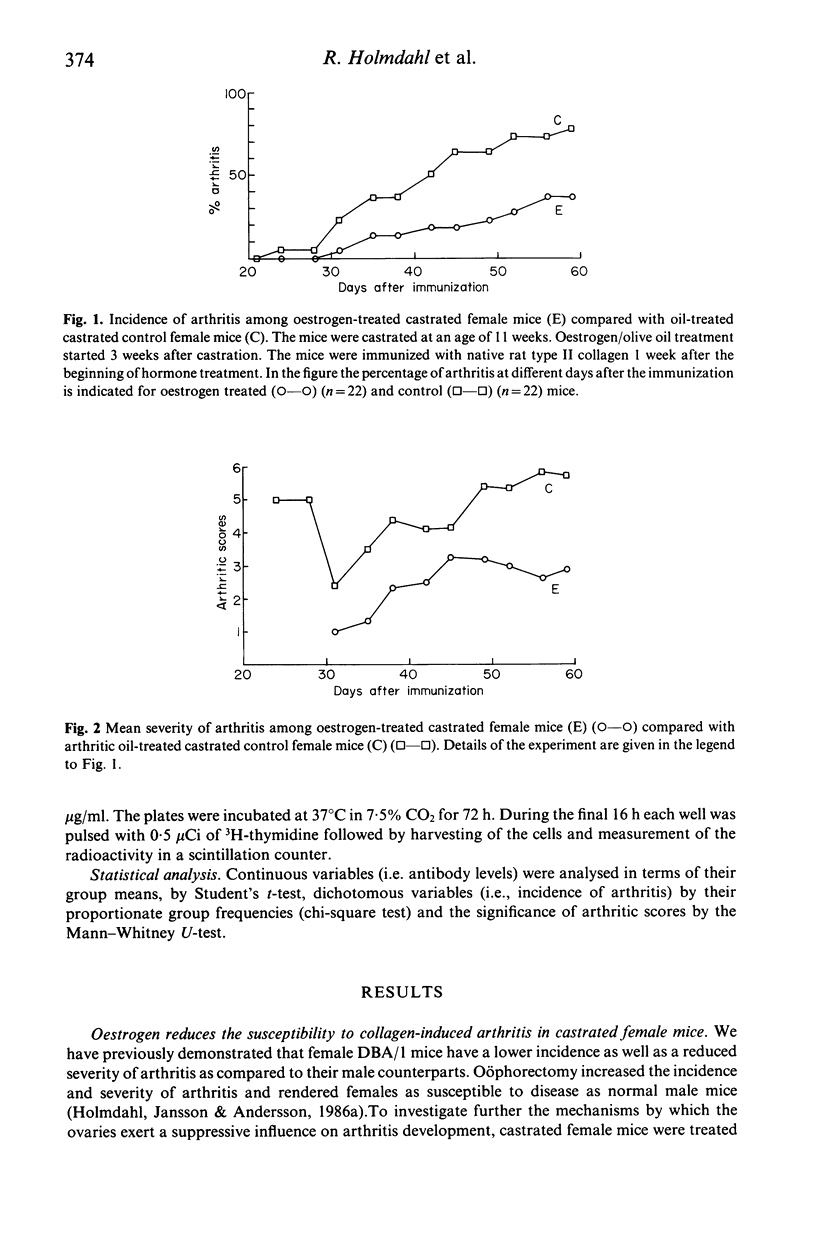
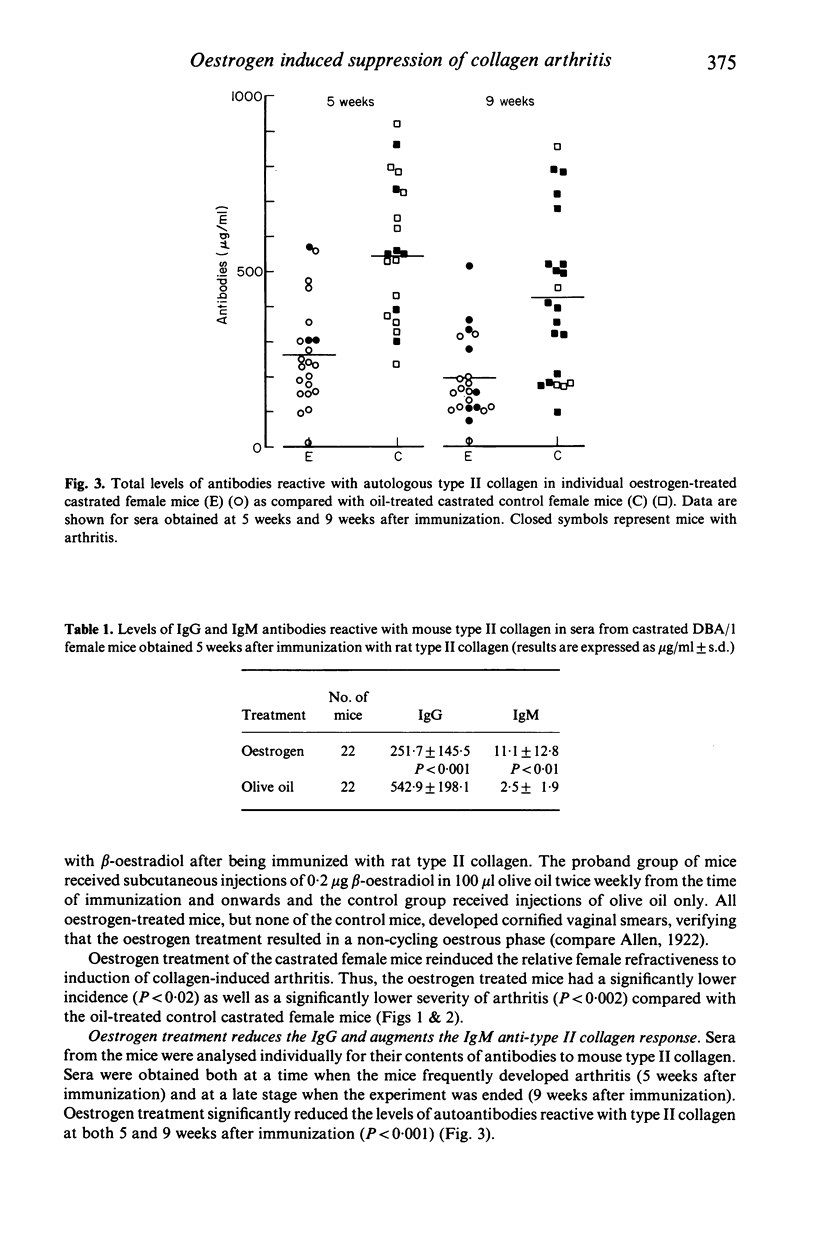
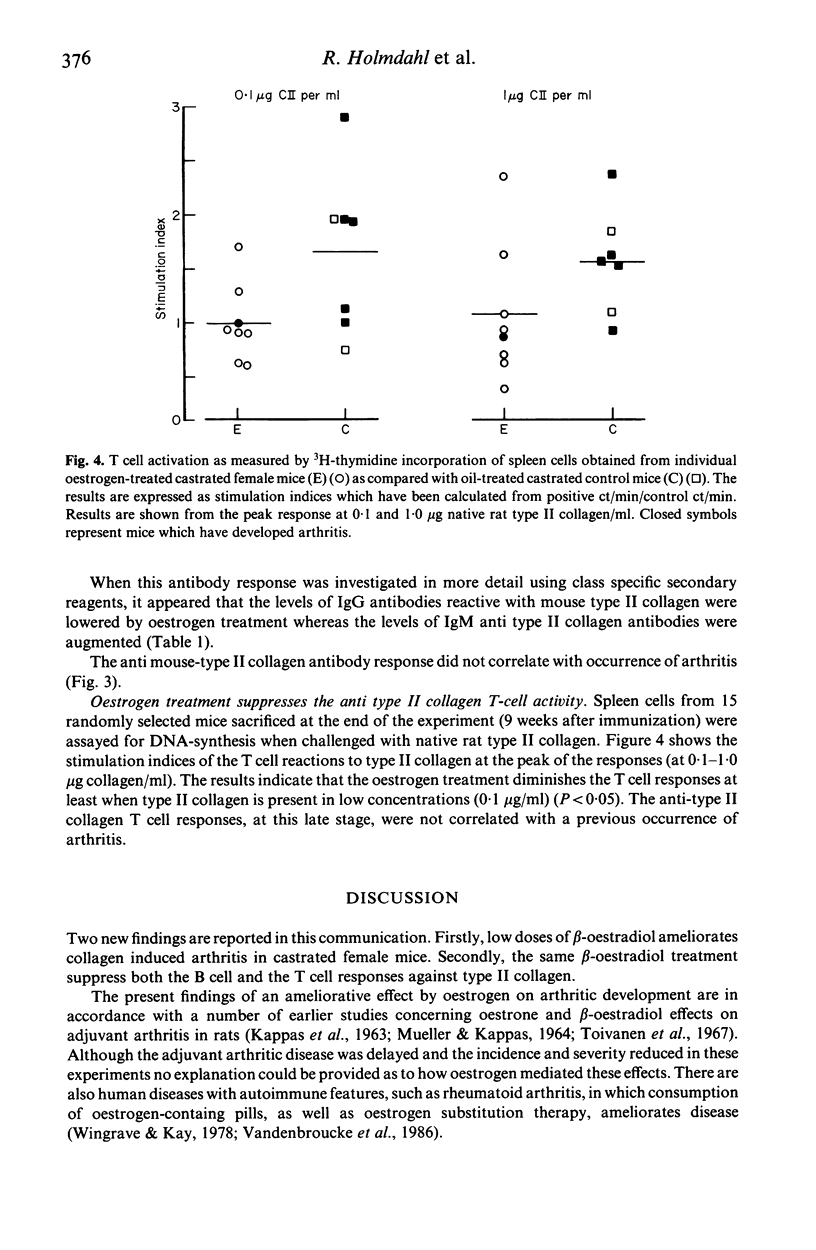
Experimental animal models can be used to help understand how oestrogen modulates autoimmune arthritis. We have previously shown that castration of female DBA/1 mice exaggerates arthritis induced with type II collagen. This report shows that treatment of castrated DBA/1 mice with low doses (0.2 micrograms twice a week) of beta-oestradiol reduces the incidence (37% vs 78% in controls) and severity (3.9 vs 5.6 mean scores) of arthritis. Levels of IgG anti type II collagen antibodies are decreased whereas levels of IgM antibodies are increased in the beta-oestradiol treated mice. The T cell response, as measured by a 3H-thymidine assay, is reduced in the beta-oestradiol treated mice. The results suggest that treatment with low doses of beta-oestradiol exerts a suppressive effect on both development of collagen arthritis as well as T cell dependent immune reactivity towards type II collagen.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Grossman C. J. Interactions between the gonadal steroids and the immune system. Science. 1985 Jan 18;227(4684):257–261. doi: 10.1126/science.3871252. [DOI] [PubMed] [Google Scholar]
- Holmdahl R., Jansson L., Andersson M. Female sex hormones suppress development of collagen-induced arthritis in mice. Arthritis Rheum. 1986 Dec;29(12):1501–1509. doi: 10.1002/art.1780291212. [DOI] [PubMed] [Google Scholar]
- Holmdahl R., Jansson L., Larsson E., Rubin K., Klareskog L. Homologous type II collagen induces chronic and progressive arthritis in mice. Arthritis Rheum. 1986 Jan;29(1):106–113. doi: 10.1002/art.1780290114. [DOI] [PubMed] [Google Scholar]
- Holmdahl R., Klareskog L., Andersson M., Hansen C. High antibody response to autologous type II collagen is restricted to H-2q. Immunogenetics. 1986;24(2):84–89. doi: 10.1007/BF00373114. [DOI] [PubMed] [Google Scholar]
- Holmdahl R., Klareskog L., Rubin K., Larsson E., Wigzell H. T lymphocytes in collagen II-induced arthritis in mice. Characterization of arthritogenic collagen II-specific T-cell lines and clones. Scand J Immunol. 1985 Sep;22(3):295–306. doi: 10.1111/j.1365-3083.1985.tb01884.x. [DOI] [PubMed] [Google Scholar]
- Jungers P., Dougados M., Pélissier C., Kuttenn F., Tron F., Lesavre P., Bach J. F. Influence of oral contraceptive therapy on the activity of systemic lupus erythematosus. Arthritis Rheum. 1982 Jun;25(6):618–623. doi: 10.1002/art.1780250603. [DOI] [PubMed] [Google Scholar]
- MUELLER M. N., KAPPAS A. ESTROGEN PHARMACOLOGY. II. SUPPRESSION OF EXPERIMENTAL IMMUNE POLYARTHRITIS. Proc Soc Exp Biol Med. 1964 Dec;117:845–847. doi: 10.3181/00379727-117-29715. [DOI] [PubMed] [Google Scholar]
- Miller E. J. Structural studies on cartilage collagen employing limited cleavage and solubilization with pepsin. Biochemistry. 1972 Dec 19;11(26):4903–4909. doi: 10.1021/bi00776a005. [DOI] [PubMed] [Google Scholar]
- NICOL T., BILBEY D. L., CHARLES L. M., CORDINGLEY J. L., VERNON-ROBERTS B. OESTROGEN: THE NATURAL STIMULANT OF BODY DEFENCE. J Endocrinol. 1964 Oct;30:277–291. doi: 10.1677/joe.0.0300277. [DOI] [PubMed] [Google Scholar]
- Roubinian J. R., Talal N., Greenspan J. S., Goodman J. R., Siiteri P. K. Effect of castration and sex hormone treatment on survival, anti-nucleic acid antibodies, and glomerulonephritis in NZB/NZW F1 mice. J Exp Med. 1978 Jun 1;147(6):1568–1583. doi: 10.1084/jem.147.6.1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith B. D., Martin G. R., Miller E. J., Dorfman A., Swarm R. Nature of the collagen synthesized by a transplanted chondrosarcoma. Arch Biochem Biophys. 1975 Jan;166(1):181–186. doi: 10.1016/0003-9861(75)90378-1. [DOI] [PubMed] [Google Scholar]
- Steinberg A. D., Melez K. A., Raveche E. S., Reeves J. P., Boegel W. A., Smathers P. A., Taurog J. D., Weinlein L., Duvic M. Approach to the study of the role of sex hormones in autoimmunity. Arthritis Rheum. 1979 Nov;22(11):1170–1176. doi: 10.1002/art.1780221103. [DOI] [PubMed] [Google Scholar]
- Stuart J. M., Dixon F. J. Serum transfer of collagen-induced arthritis in mice. J Exp Med. 1983 Aug 1;158(2):378–392. doi: 10.1084/jem.158.2.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart J. M., Townes A. S., Kang A. H. Nature and specificity of the immune response to collagen in type II collagen-induced arthritis in mice. J Clin Invest. 1982 Mar;69(3):673–683. doi: 10.1172/JCI110495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenbroucke J. P., Valkenburg H. A., Boersma J. W., Cats A., Festen J. J., Huber-Bruning O., Rasker J. J. Oral contraceptives and rheumatoid arthritis: further evidence for a preventive effect. Lancet. 1982 Oct 16;2(8303):839–842. doi: 10.1016/s0140-6736(82)90809-1. [DOI] [PubMed] [Google Scholar]
- Vandenbroucke J. P., Witteman J. C., Valkenburg H. A., Boersma J. W., Cats A., Festen J. J., Hartman A. P., Huber-Bruning O., Rasker J. J., Weber J. Noncontraceptive hormones and rheumatoid arthritis in perimenopausal and postmenopausal women. JAMA. 1986 Mar 14;255(10):1299–1303. [PubMed] [Google Scholar]



