Abstract
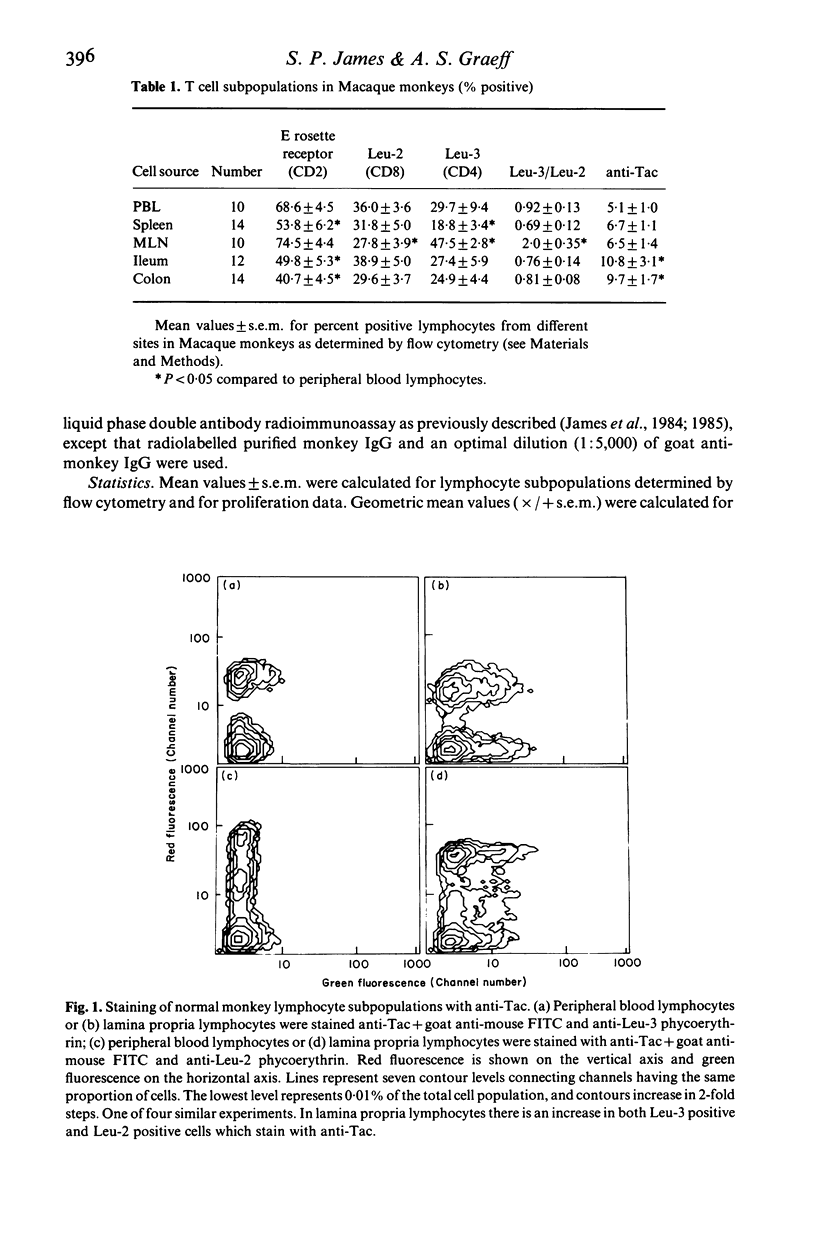
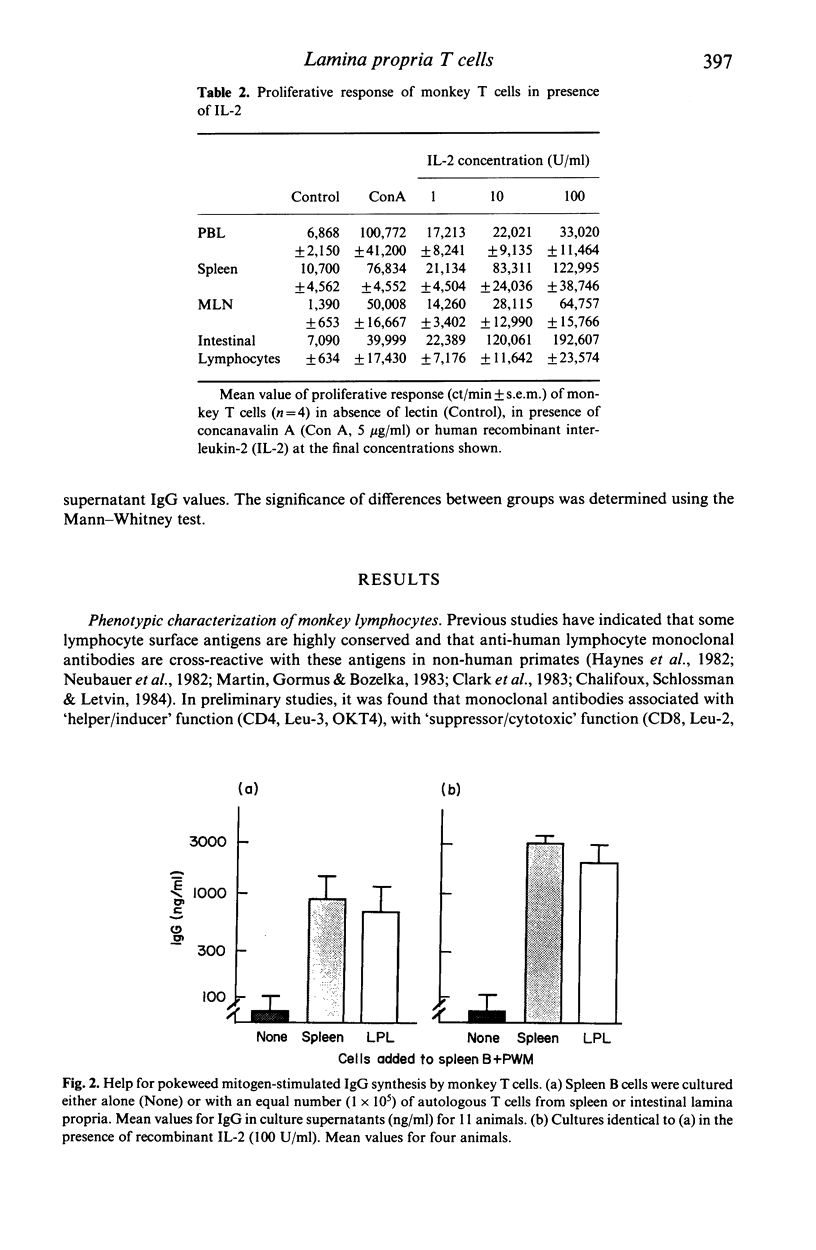
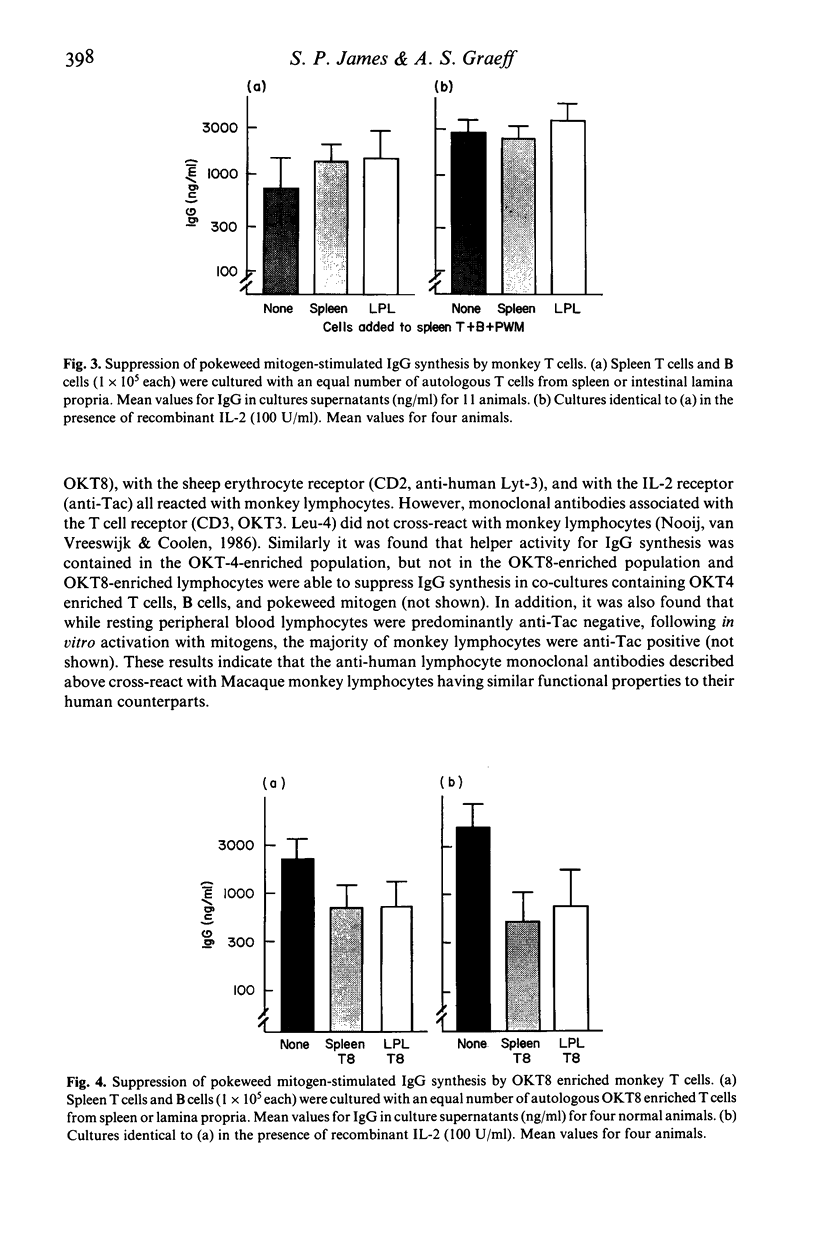
The role of T cell activation on the immunoregulatory function of lymphocytes in the lamina propria of the normal intestine was investigated. Lymphocytes were isolated from different sites in non-human primates, and their cell surface phenotypes, response to exogenous IL-2, and immunoregulatory function in pokeweed mitogen stimulated cultures were determined. The proportions of Leu-3+ (CD4) and Leu-2+ (CD8) lymphocytes in isolated lamina propria cells were similar to peripheral blood and spleen, but the proportion of Leu-3+ cells was significantly higher in mesenteric lymph node lymphocytes. The proportion of IL-2 receptor positive cells was significantly higher in lamina propria compared with peripheral blood, spleen and mesenteric lymph nodes. Increased IL-2 receptor expression was found on both Leu-3+ and Leu-2+ lamina propria T cells. In addition, although lamina propria T cells had a lower proliferative response to Con A, they had a significantly higher response when cultured with recombinant IL-2, indicating that they have increased expression of functional IL-2 receptors. The helper function of lamina propria T cells for pokeweed mitogen stimulated immunoglobulin synthesis was enhanced by recombinant IL-2. Although Leu-2+ lymphocytes in the lamina propria had increased IL-2 receptor expression, suppressor function of lamina propria T cells was similar to that of spleen cells, and was not enhanced by addition of exogenous IL-2. Thus, T cells in the lamina propria have increased expression of functional IL-2 receptors, and recombinant IL-2 enhances the helper, but not the suppressor function of lamina propria T cells for immunoglobulin synthesis.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chalifoux L. V., Schlossman S. F., Letvin N. L. Delineation of lymphocyte subsets in lymph nodes of nonhuman primates. Clin Immunol Immunopathol. 1984 Apr;31(1):96–101. doi: 10.1016/0090-1229(84)90193-4. [DOI] [PubMed] [Google Scholar]
- Clark E. A., Martin P. J., Hansen J. A., Ledbetter J. A. Evolution of epitopes on human and nonhuman primate lymphocyte cell surface antigens. Immunogenetics. 1983;18(6):599–615. doi: 10.1007/BF00345968. [DOI] [PubMed] [Google Scholar]
- Elson C. O., Machelski E., Weiserbs D. B. T cell-B cell regulation in the intestinal lamina propria in Crohn's disease. Gastroenterology. 1985 Aug;89(2):321–327. doi: 10.1016/0016-5085(85)90332-4. [DOI] [PubMed] [Google Scholar]
- Fiocchi C., Battisto J. R., Farmer R. G. Studies on isolated gut mucosal lymphocytes in inflammatory bowel disease. Detection of activated T cells and enhanced proliferation to Staphylococcus aureus and lipopolysaccharides. Dig Dis Sci. 1981 Aug;26(8):728–736. doi: 10.1007/BF01316863. [DOI] [PubMed] [Google Scholar]
- Fiocchi C., Hilfiker M. L., Youngman K. R., Doerder N. C., Finke J. H. Interleukin 2 activity of human intestinal mucosa mononuclear cells. Decreased levels in inflammatory bowel disease. Gastroenterology. 1984 Apr;86(4):734–742. [PubMed] [Google Scholar]
- Haynes B. F., Dowell D. L., Hensley L. L., Gore I., Metzgar R. S. Human T cell antigen expression by primate T cells. Science. 1982 Jan 15;215(4530):298–300. doi: 10.1126/science.6171885. [DOI] [PubMed] [Google Scholar]
- James S. P., Fiocchi C., Graeff A. S., Strober W. Immunoregulatory function of lamina propria T cells in Crohn's disease. Gastroenterology. 1985 May;88(5 Pt 1):1143–1150. doi: 10.1016/s0016-5085(85)80073-1. [DOI] [PubMed] [Google Scholar]
- James S. P., Fiocchi C., Graeff A. S., Strober W. Phenotypic analysis of lamina propria lymphocytes. Predominance of helper-inducer and cytolytic T-cell phenotypes and deficiency of suppressor-inducer phenotypes in Crohn's disease and control patients. Gastroenterology. 1986 Dec;91(6):1483–1489. [PubMed] [Google Scholar]
- James S. P., Graeff A. S. Spontaneous and lymphokine-induced cytotoxic activity of monkey intestinal mucosal lymphocytes. Cell Immunol. 1985 Jul;93(2):387–397. doi: 10.1016/0008-8749(85)90143-1. [DOI] [PubMed] [Google Scholar]
- James S. P., Neckers L. M., Graeff A. S., Cossman J., Balch C. M., Strober W. Suppression of immunoglobulin synthesis by lymphocyte subpopulations in patients with Crohn's disease. Gastroenterology. 1984 Jun;86(6):1510–1518. [PubMed] [Google Scholar]
- Kansas G. S., Wood G. S., Fishwild D. M., Engleman E. G. Functional characterization of human T lymphocyte subsets distinguished by monoclonal anti-leu-8. J Immunol. 1985 May;134(5):2995–3002. [PubMed] [Google Scholar]
- Kawanishi H., Saltzman L. E., Strober W. Mechanisms regulating IgA class-specific immunoglobulin production in murine gut-associated lymphoid tissues. I. T cells derived from Peyer's patches that switch sIgM B cells to sIgA B cells in vitro. J Exp Med. 1983 Feb 1;157(2):433–450. doi: 10.1084/jem.157.2.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyono H., McGhee J. R., Mosteller L. M., Eldridge J. H., Koopman W. J., Kearney J. F., Michalek S. M. Murine Peyer's patch T cell clones. Characterization of antigen-specific helper T cells for immunoglobulin A responses. J Exp Med. 1982 Oct 1;156(4):1115–1130. doi: 10.1084/jem.156.4.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDermott R. P., Nash G. S., Bertovich M. J., Seiden M. V., Bragdon M. J., Beale M. G. Alterations of IgM, IgG, and IgA Synthesis and secretion by peripheral blood and intestinal mononuclear cells from patients with ulcerative colitis and Crohn's disease. Gastroenterology. 1981 Nov;81(5):844–852. [PubMed] [Google Scholar]
- Martin L. N., Gormus B. J., Bozelka B. E. Functional analysis of monkey lymphocyte subsets defined by OKT4 and OKT8 Monoclonal Antibodies. Cell Immunol. 1983 Apr 15;77(2):338–347. doi: 10.1016/0008-8749(83)90034-5. [DOI] [PubMed] [Google Scholar]
- Neubauer R. H., Marchalonis J. J., Strnad B. C., Rabin H. Surface markers of primate B and T lymphoid cell lines identified by antibodies to human and simian lymphocyte antigens. J Immunogenet. 1982 Aug;9(4):209–221. doi: 10.1111/j.1744-313x.1982.tb00976.x. [DOI] [PubMed] [Google Scholar]
- Nooij F. J., van Vreeswijk W., Coolen J. Polymorphism for RhT3, a CD3-like cell surface antigen, expressed on rhesus monkey T lymphocytes. Immunology. 1986 Dec;59(4):611–620. [PMC free article] [PubMed] [Google Scholar]
- Selby W. S., Janossy G., Bofill M., Jewell D. P. Intestinal lymphocyte subpopulations in inflammatory bowel disease: an analysis by immunohistological and cell isolation techniques. Gut. 1984 Jan;25(1):32–40. doi: 10.1136/gut.25.1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith K. A., Cantrell D. A. Interleukin 2 regulates its own receptors. Proc Natl Acad Sci U S A. 1985 Feb;82(3):864–868. doi: 10.1073/pnas.82.3.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchiyama T., Broder S., Waldmann T. A. A monoclonal antibody (anti-Tac) reactive with activated and functionally mature human T cells. I. Production of anti-Tac monoclonal antibody and distribution of Tac (+) cells. J Immunol. 1981 Apr;126(4):1393–1397. [PubMed] [Google Scholar]


