Abstract
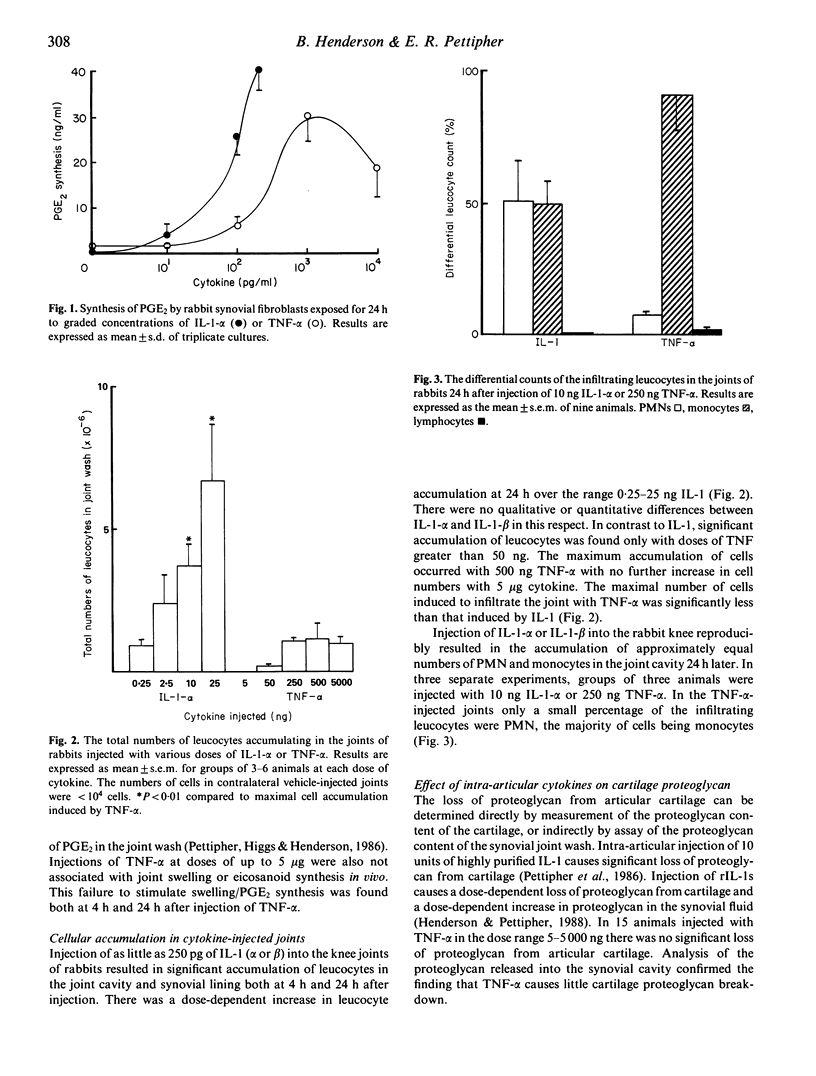
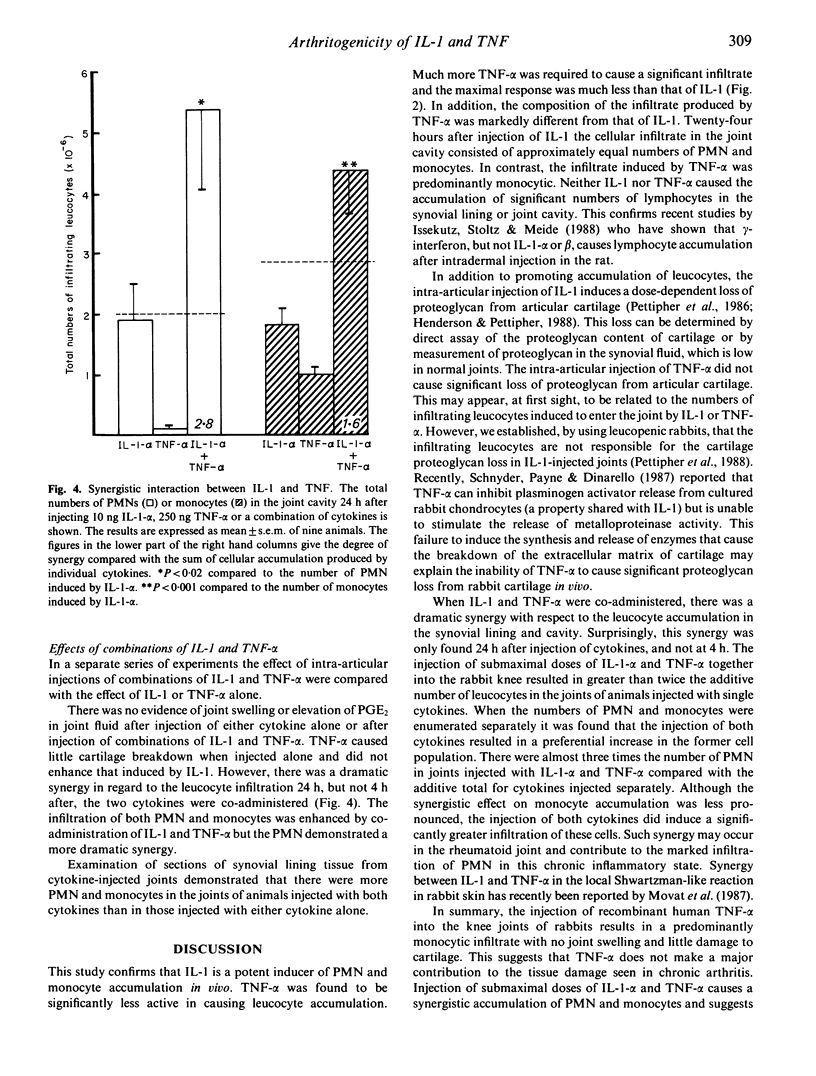
Intra-articular injection of highly purified or recombinant interleukin 1 (IL-1) into the rabbit knee induces a transient synovitis with leucocytic infiltration into the synovial lining and joint cavity and loss of proteoglycan from articular cartilage. Tumour necrosis factor alpha (TNF-alpha), which has many of the actions of IL-1, in the dose range 50-5,000 ng induced infiltration of leucocytes into the joint but failed to cause significant proteoglycan loss from cartilage. The nature of the leucocytic infiltrate induced by intra-articular TNF-alpha was predominantly monocytic compared with the mixed polymorphonuclear (PMN)/monocytic infiltrate induced by IL-1. Neither cytokine induced the accumulation of significant numbers of lymphocytes. In addition, on a molar basis, TNF-alpha was significantly less active than IL-1 in causing cell accumulation in the joint. Injection of submaximal doses of IL-1 and TNF into the rabbit resulted in a marked synergy with respect to the accumulation of PMN. The conclusion from these studies is that TNF-alpha could contribute to the PMN accumulation in the human joint in rheumatoid arthritis but is unlikely to be important in the destruction of articular cartilage.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bertolini D. R., Nedwin G. E., Bringman T. S., Smith D. D., Mundy G. R. Stimulation of bone resorption and inhibition of bone formation in vitro by human tumour necrosis factors. Nature. 1986 Feb 6;319(6053):516–518. doi: 10.1038/319516a0. [DOI] [PubMed] [Google Scholar]
- Bevilacqua M. P., Pober J. S., Mendrick D. L., Cotran R. S., Gimbrone M. A., Jr Identification of an inducible endothelial-leukocyte adhesion molecule. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9238–9242. doi: 10.1073/pnas.84.24.9238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevilacqua M. P., Pober J. S., Wheeler M. E., Cotran R. S., Gimbrone M. A., Jr Interleukin 1 acts on cultured human vascular endothelium to increase the adhesion of polymorphonuclear leukocytes, monocytes, and related leukocyte cell lines. J Clin Invest. 1985 Nov;76(5):2003–2011. doi: 10.1172/JCI112200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavender D. E., Haskard D. O., Joseph B., Ziff M. Interleukin 1 increases the binding of human B and T lymphocytes to endothelial cell monolayers. J Immunol. 1986 Jan;136(1):203–207. [PubMed] [Google Scholar]
- Dayer J. M., Beutler B., Cerami A. Cachectin/tumor necrosis factor stimulates collagenase and prostaglandin E2 production by human synovial cells and dermal fibroblasts. J Exp Med. 1985 Dec 1;162(6):2163–2168. doi: 10.1084/jem.162.6.2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Giovine F. S., Nuki G., Duff G. W. Tumour necrosis factor in synovial exudates. Ann Rheum Dis. 1988 Sep;47(9):768–772. doi: 10.1136/ard.47.9.768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias J. A., Gustilo K., Baeder W., Freundlich B. Synergistic stimulation of fibroblast prostaglandin production by recombinant interleukin 1 and tumor necrosis factor. J Immunol. 1987 Jun 1;138(11):3812–3816. [PubMed] [Google Scholar]
- Farndale R. W., Buttle D. J., Barrett A. J. Improved quantitation and discrimination of sulphated glycosaminoglycans by use of dimethylmethylene blue. Biochim Biophys Acta. 1986 Sep 4;883(2):173–177. doi: 10.1016/0304-4165(86)90306-5. [DOI] [PubMed] [Google Scholar]
- Farndale R. W., Sayers C. A., Barrett A. J. A direct spectrophotometric microassay for sulfated glycosaminoglycans in cartilage cultures. Connect Tissue Res. 1982;9(4):247–248. doi: 10.3109/03008208209160269. [DOI] [PubMed] [Google Scholar]
- Fell H. B., Jubb R. W. The effect of synovial tissue on the breakdown of articular cartilage in organ culture. Arthritis Rheum. 1977 Sep-Oct;20(7):1359–1371. doi: 10.1002/art.1780200710. [DOI] [PubMed] [Google Scholar]
- Figari I. S., Mori N. A., Palladino M. A., Jr Regulation of neutrophil migration and superoxide production by recombinant tumor necrosis factors-alpha and -beta: comparison to recombinant interferon-gamma and interleukin-1 alpha. Blood. 1987 Oct;70(4):979–984. [PubMed] [Google Scholar]
- Gamble J. R., Harlan J. M., Klebanoff S. J., Vadas M. A. Stimulation of the adherence of neutrophils to umbilical vein endothelium by human recombinant tumor necrosis factor. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8667–8671. doi: 10.1073/pnas.82.24.8667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgilis K., Schaefer C., Dinarello C. A., Klempner M. S. Human recombinant interleukin 1 beta has no effect on intracellular calcium or on functional responses of human neutrophils. J Immunol. 1987 May 15;138(10):3403–3407. [PubMed] [Google Scholar]
- Henderson B., Pettipher E. R. Comparison of the in vivo inflammatory activities after intra-articular injection of natural and recombinant IL-1 alpha and IL-1 beta in the rabbit. Biochem Pharmacol. 1988 Nov 1;37(21):4171–4176. doi: 10.1016/0006-2952(88)90112-8. [DOI] [PubMed] [Google Scholar]
- Henderson B., Pettipher E. R., Higgs G. A. Mediators of rheumatoid arthritis. Br Med Bull. 1987 Apr;43(2):415–428. doi: 10.1093/oxfordjournals.bmb.a072191. [DOI] [PubMed] [Google Scholar]
- Issekutz T. B., Stoltz J. M., vd Meide P. Lymphocyte recruitment in delayed-type hypersensitivity. The role of IFN-gamma. J Immunol. 1988 May 1;140(9):2989–2993. [PubMed] [Google Scholar]
- Luger T. A., Charon J. A., Colot M., Micksche M., Oppenheim J. J. Chemotactic properties of partially purified human epidermal cell-derived thymocyte-activating factor (ETAF) for polymorphonuclear and mononuclear cells. J Immunol. 1983 Aug;131(2):816–820. [PubMed] [Google Scholar]
- Ming W. J., Bersani L., Mantovani A. Tumor necrosis factor is chemotactic for monocytes and polymorphonuclear leukocytes. J Immunol. 1987 Mar 1;138(5):1469–1474. [PubMed] [Google Scholar]
- Miossec P., Yu C. L., Ziff M. Lymphocyte chemotactic activity of human interleukin 1. J Immunol. 1984 Oct;133(4):2007–2011. [PubMed] [Google Scholar]
- Movat H. Z., Burrowes C. E., Cybulsky M. I., Dinarello C. A. Acute inflammation and a Shwartzman-like reaction induced by interleukin-1 and tumor necrosis factor. Synergistic action of the cytokines in the induction of inflammation and microvascular injury. Am J Pathol. 1987 Dec;129(3):463–476. [PMC free article] [PubMed] [Google Scholar]
- Pettipher E. R., Henderson B., Moncada S., Higgs G. A. Leucocyte infiltration and cartilage proteoglycan loss in immune arthritis in the rabbit. Br J Pharmacol. 1988 Sep;95(1):169–176. doi: 10.1111/j.1476-5381.1988.tb16561.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettipher E. R., Higgs G. A., Henderson B. Interleukin 1 induces leukocyte infiltration and cartilage proteoglycan degradation in the synovial joint. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8749–8753. doi: 10.1073/pnas.83.22.8749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saklatvala J., Pilsworth L. M., Sarsfield S. J., Gavrilovic J., Heath J. K. Pig catabolin is a form of interleukin 1. Cartilage and bone resorb, fibroblasts make prostaglandin and collagenase, and thymocyte proliferation is augmented in response to one protein. Biochem J. 1984 Dec 1;224(2):461–466. doi: 10.1042/bj2240461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saklatvala J. Tumour necrosis factor alpha stimulates resorption and inhibits synthesis of proteoglycan in cartilage. Nature. 1986 Aug 7;322(6079):547–549. doi: 10.1038/322547a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmon J. A. A radioimmunoassay for 6-keto-prostaglandin F1alpha. Prostaglandins. 1978 Mar;15(3):383–397. doi: 10.1016/0090-6980(78)90122-3. [DOI] [PubMed] [Google Scholar]
- Schnyder J., Payne T., Dinarello C. A. Human monocyte or recombinant interleukin 1's are specific for the secretion of a metalloproteinase from chondrocytes. J Immunol. 1987 Jan 15;138(2):496–503. [PubMed] [Google Scholar]
- Stashenko P., Dewhirst F. E., Peros W. J., Kent R. L., Ago J. M. Synergistic interactions between interleukin 1, tumor necrosis factor, and lymphotoxin in bone resorption. J Immunol. 1987 Mar 1;138(5):1464–1468. [PubMed] [Google Scholar]


