Abstract
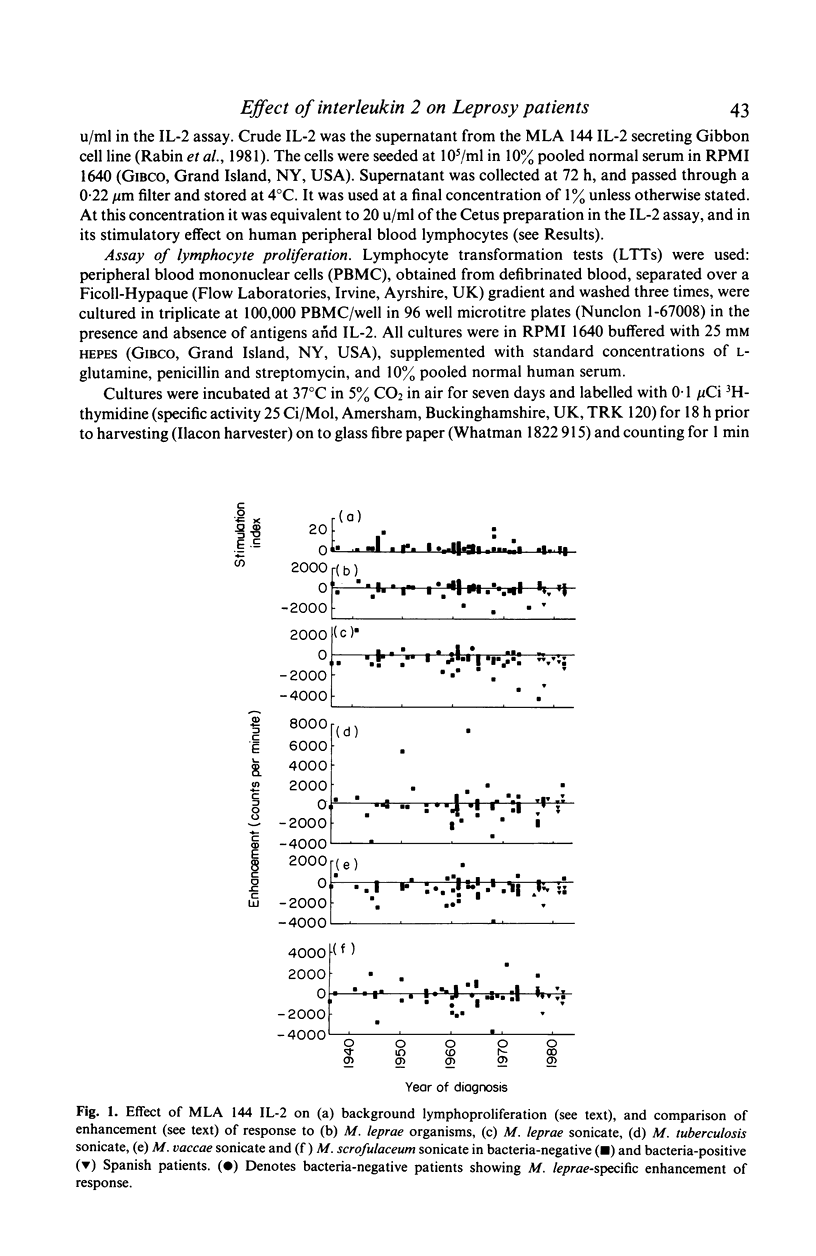
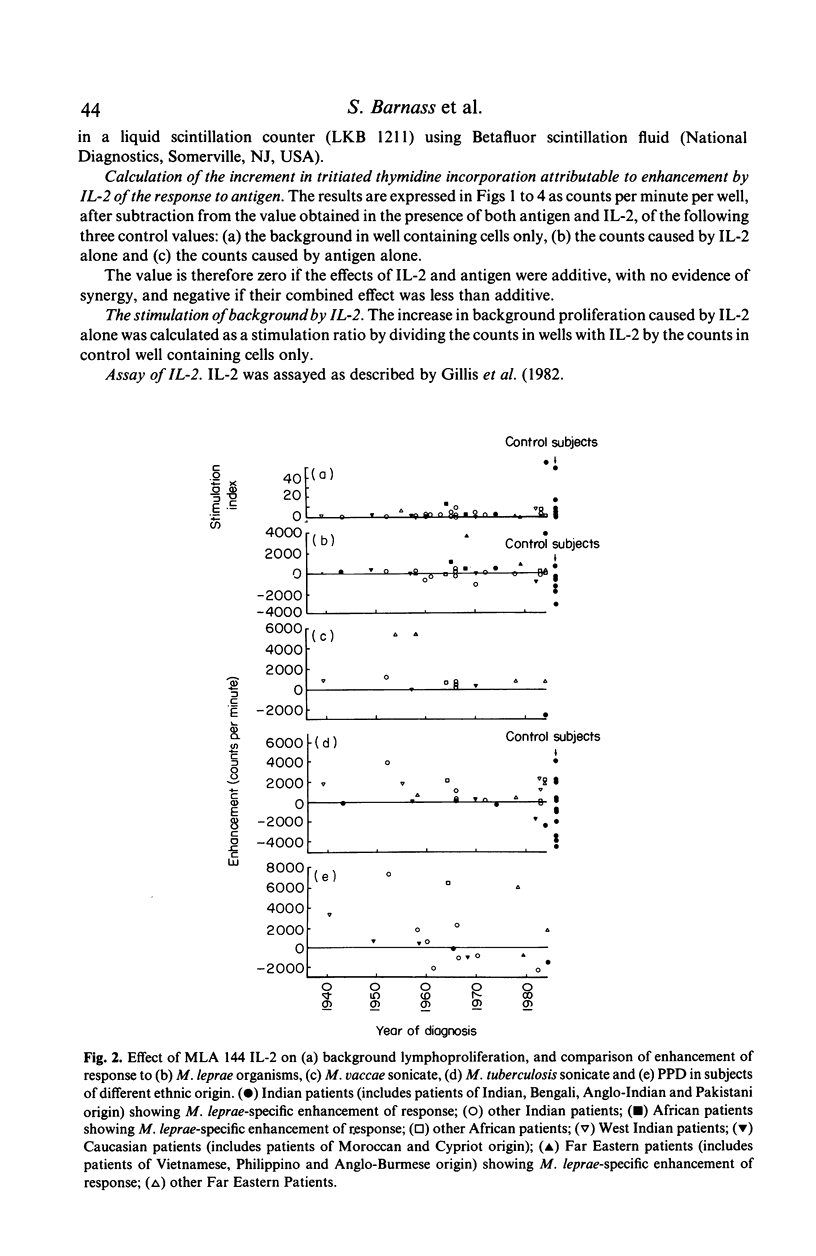
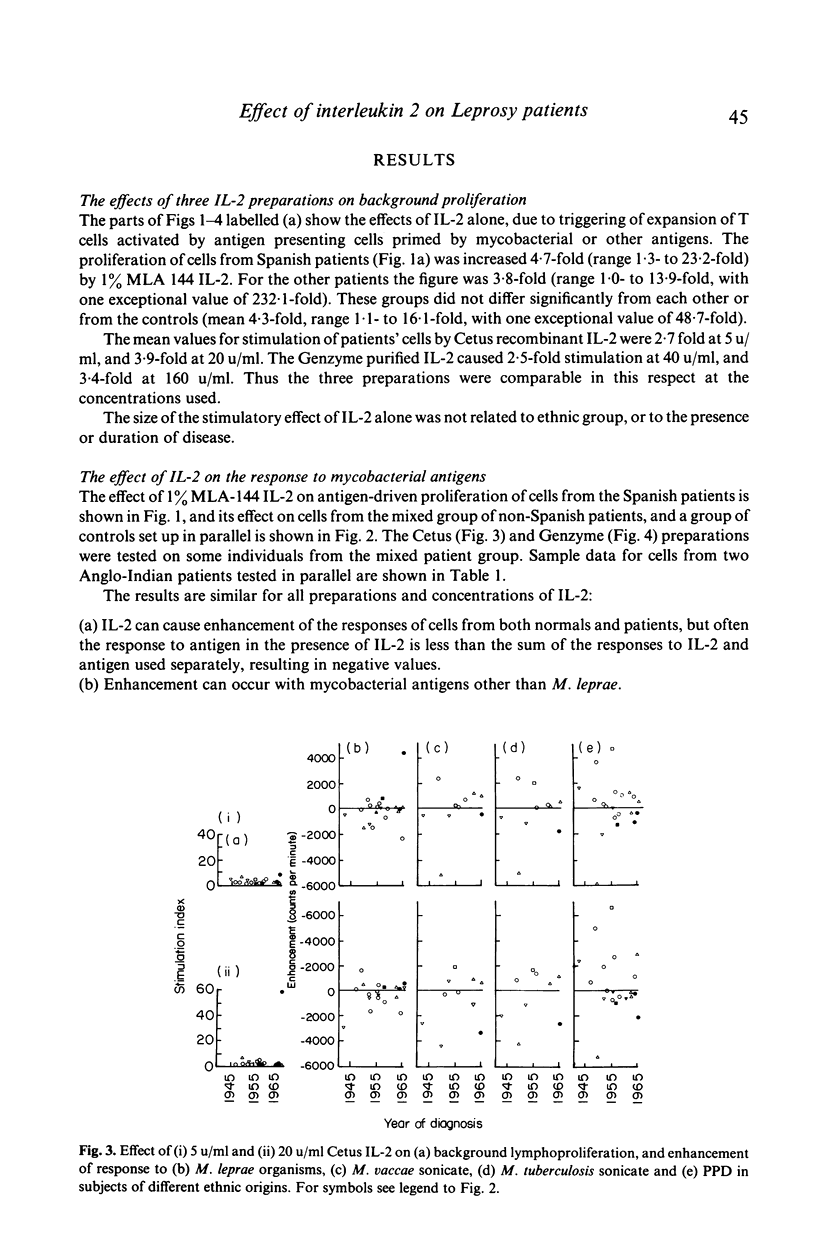
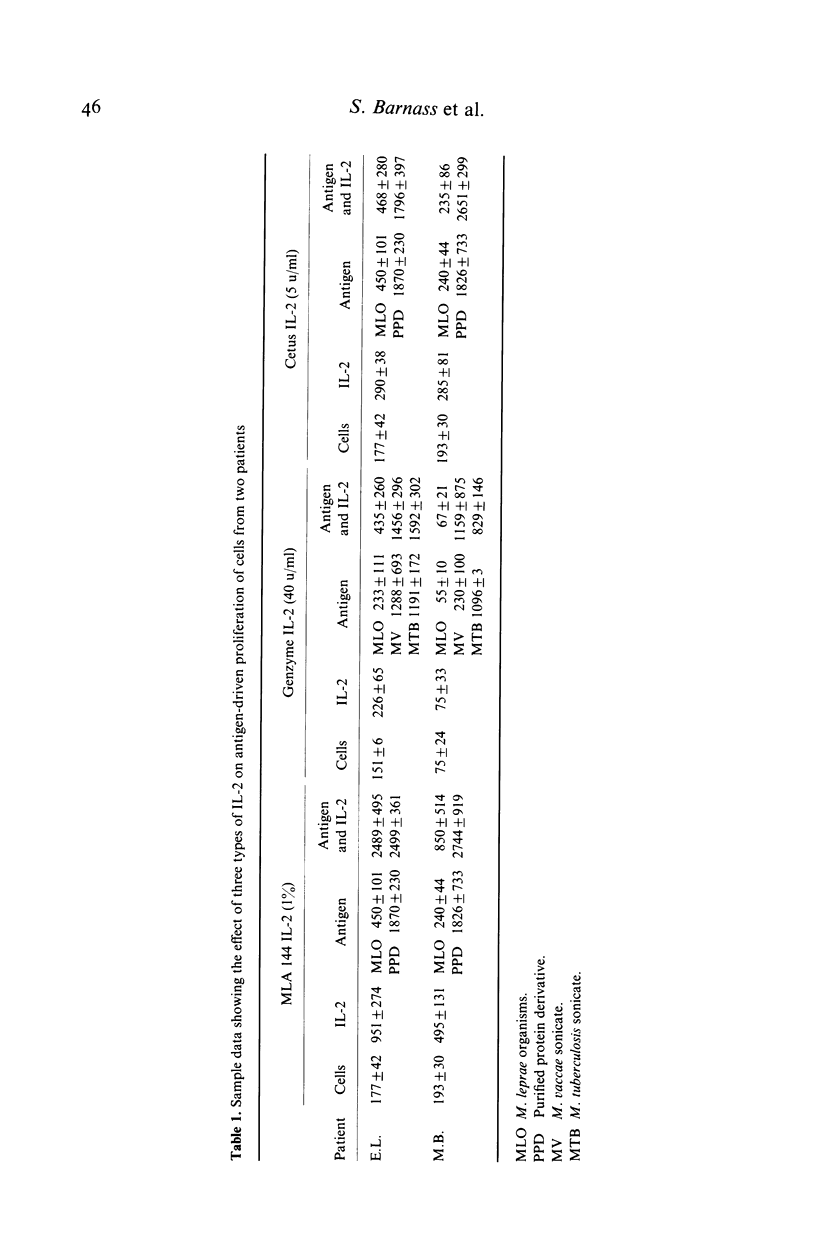
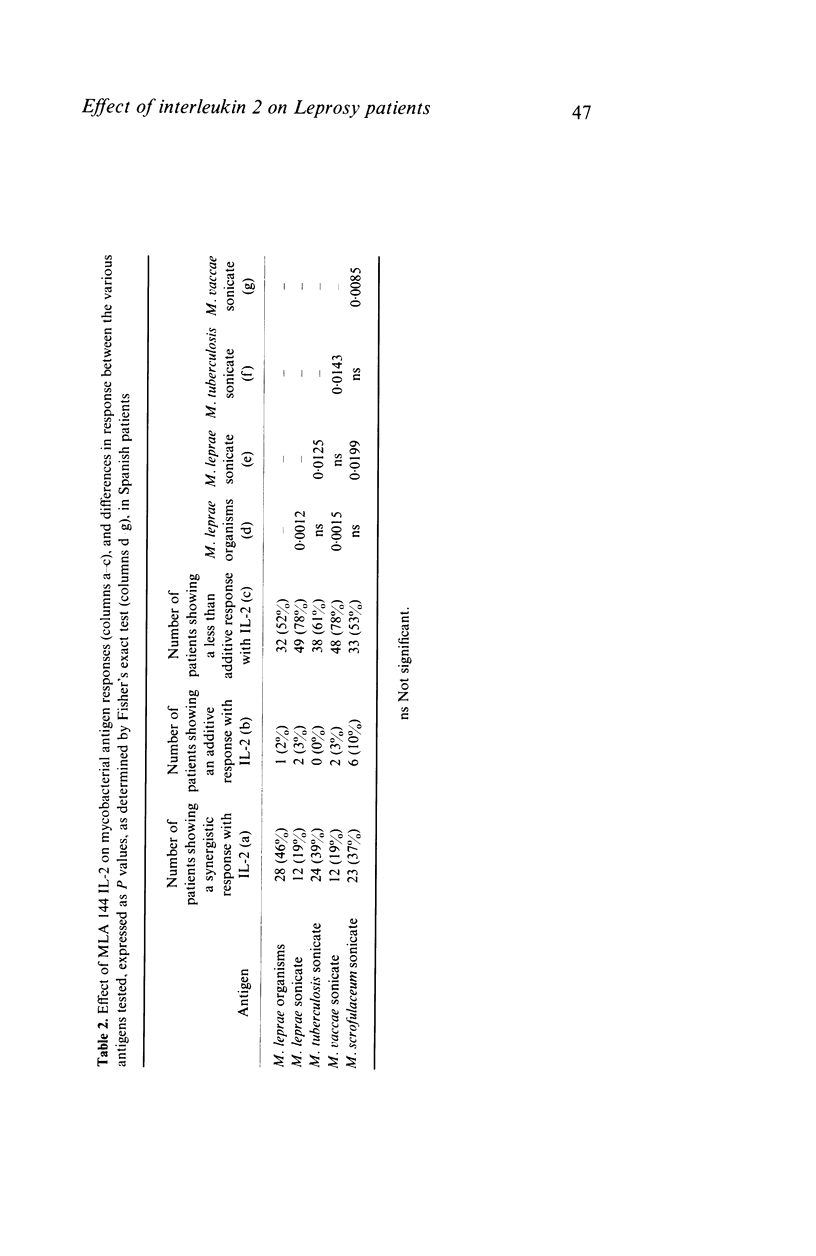
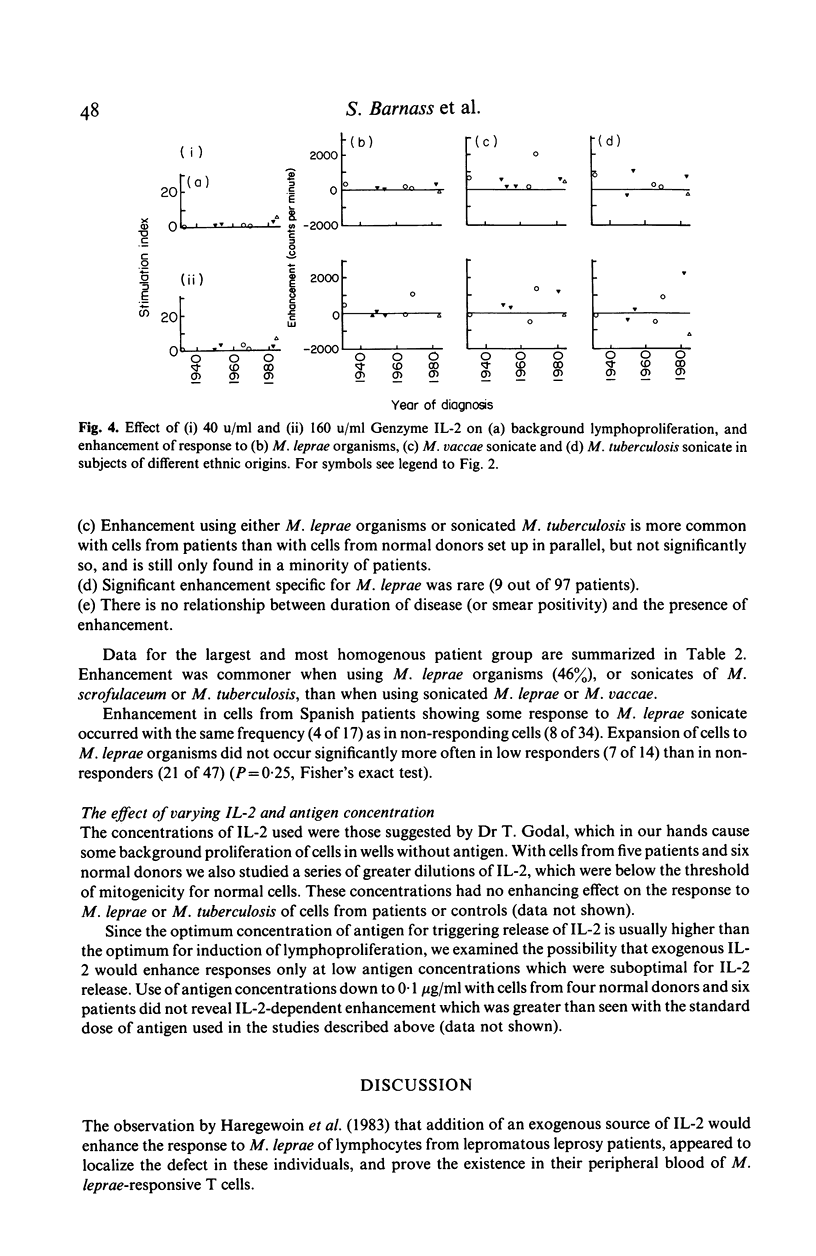
Peripheral blood mononuclear cells from 97 predominantly lepromatous leprosy patients and 11 control subjects were tested in a lymphoproliferative assay for response to Mycobacterium leprae (whole and sonicated), and sonicated M. vaccae, M. tuberculosis, and M. scrofulaceum, in the presence and absence of three types of interleukin 2 (IL-2) (crude, purified, and recombinant). IL-2 enhanced the response to sonicated M. tuberculosis and M. leprae organisms more often in patients than in control subjects, but not significantly so and only in a minority of patients. This effect was significantly more common (though still only found in a minority of 46%) using M. leprae organisms as antigen, than when using sonicates of M. leprae (19%) or M. vaccae (19%). However it was nearly as frequent using sonicated M. tuberculosis, or M. scrofulaceum. Thus in only nine patients was the effect specific to M. leprae. Enhancement by IL-2 could not be related to the type of IL-2 used, the dose of antigen, or the amount of endogenous IL-2 released by the cells tested. Similarly it was not related to the extent to which IL-2 caused increased background proliferation in control wells, which occurred to an equal extent using cells from control subjects, nor was it related to the extent of antigen-driven proliferation. The data have also been analysed in relation to duration of disease (50 years to a few weeks) and ethnic origin. No correlations have been revealed. Thus enhancement by IL-2 of the lymphoproliferative response to mycobacterial antigens does occur using cells from lepromatous leprosy patients, but it is found in a minority of patients, it is not specific to M. leprae, and can occur with cells from normal donors.
Full text
PDF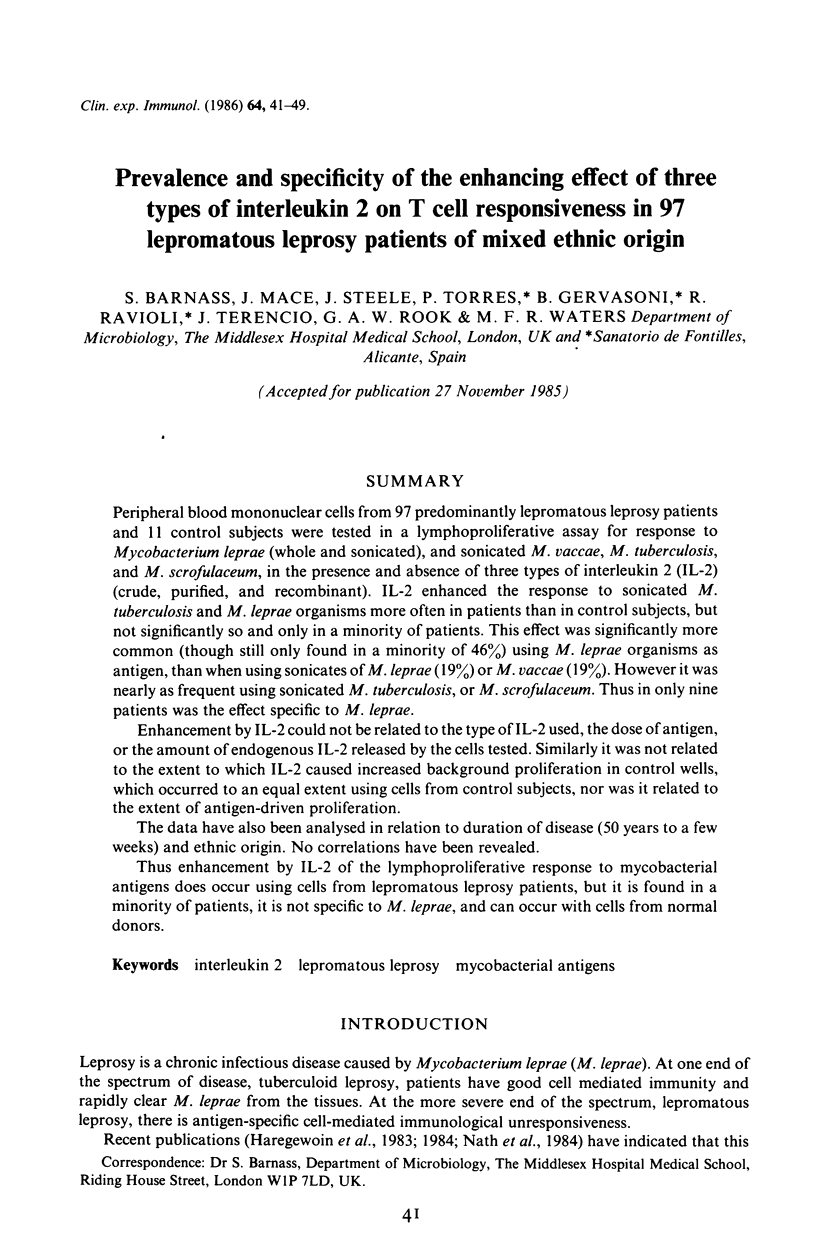


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Emery P., Panayi G. S., Nouri A. M. Interleukin-2 reverses deficient cell-mediated immune responses in rheumatoid arthritis. Clin Exp Immunol. 1984 Jul;57(1):123–129. [PMC free article] [PubMed] [Google Scholar]
- Gastl G. A., Feldmeier H., Doehring E., Kortmann C., Daffalla A. A., Peter H. H. Numerical and functional alterations of lymphocytes in human schistosomiasis. Scand J Immunol. 1984 May;19(5):469–479. doi: 10.1111/j.1365-3083.1984.tb00956.x. [DOI] [PubMed] [Google Scholar]
- Gillis S., Mochizuki D. Y., Conlon P. J., Hefeneider S. H., Ramthun C. A., Gillis A. E., Frank M. B., Henney C. S., Watson J. D. Molecular characterization of interleukin 2. Immunol Rev. 1982;63:167–209. doi: 10.1111/j.1600-065x.1982.tb00415.x. [DOI] [PubMed] [Google Scholar]
- Haregewoin A., Godal T., Mustafa A. S., Belehu A., Yemaneberhan T. T-cell conditioned media reverse T-cell unresponsiveness in lepromatous leprosy. Nature. 1983 May 26;303(5915):342–344. doi: 10.1038/303342a0. [DOI] [PubMed] [Google Scholar]
- Haregewoin A., Mustafa A. S., Helle I., Waters M. F., Leiker D. L., Godal T. Reversal by interleukin-2 of the T cell unresponsiveness of lepromatous leprosy to Mycobacterium leprae. Immunol Rev. 1984 Aug;80:77–86. doi: 10.1111/j.1600-065x.1984.tb00495.x. [DOI] [PubMed] [Google Scholar]
- Kaplan G., Weinstein D. E., Steinman R. M., Levis W. R., Elvers U., Patarroyo M. E., Cohn Z. A. An analysis of in vitro T cell responsiveness in lepromatous leprosy. J Exp Med. 1985 Sep 1;162(3):917–929. doi: 10.1084/jem.162.3.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohagheghpour N., Gelber R. H., Larrick J. W., Sasaki D. T., Brennan P. J., Engleman E. G. Defective cell-mediated immunity in leprosy: failure of T cells from lepromatous leprosy patients to respond to Mycobacterium leprae is associated with defective expression of interleukin 2 receptors and is not reconstituted by interleukin 2. J Immunol. 1985 Aug;135(2):1443–1449. [PubMed] [Google Scholar]
- Nath I., Sathish M., Jayaraman T., Bhutani L. K., Sharma A. K. Evidence for the presence of M. leprae reactive T lymphocytes in patients with lepromatous leprosy. Clin Exp Immunol. 1984 Dec;58(3):522–530. [PMC free article] [PubMed] [Google Scholar]
- Ottenhoff T. H., Elferink D. G., de Vries R. R. Unresponsiveness to Mycobacterium leprae in lepromatous leprosy in vitro: reversible or not? Int J Lepr Other Mycobact Dis. 1984 Sep;52(3):419–424. [PubMed] [Google Scholar]
- Paul R. C., Stanford J. L., Carswell J. W. Multiple skin testing in leprosy. J Hyg (Lond) 1975 Aug;75(1):57–68. doi: 10.1017/s0022172400047069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabin H., Hopkins R. F., 3rd, Ruscetti F. W., Neubauer R. H., Brown R. L., Kawakami T. G. Spontaneous release of a factor with properties of T cell growth factor from a continuous line of primate tumor T cells. J Immunol. 1981 Nov;127(5):1852–1856. [PubMed] [Google Scholar]
- Ridley D. S., Jopling W. H. Classification of leprosy according to immunity. A five-group system. Int J Lepr Other Mycobact Dis. 1966 Jul-Sep;34(3):255–273. [PubMed] [Google Scholar]


