Abstract
Abnormal ratios of T helper-type to T suppressor-type lymphocytes in the blood of patients with replanted autologous splenic tissue led to the present study in rats. Lymphocyte subsets were studied in the blood, mesenteric lymph nodes and spleen after autotransplantation and compared to splenectomized and control rats. In the blood of transplanted rats the percentage of T and T helper-type lymphocytes was lower, in the spleen B lymphocytes higher and T lymphocytes and their subsets lower. Comparable changes were seen in the lymph nodes. The data of the mesenteric lymph nodes in autotransplanted rats did not differ from splenectomized animals. Even after 37 weeks the regenerated splenic tissue only reached 13% of the weight of control rats and the absolute lymphocyte number was only 2.5% of a normal spleen. Splenic autotransplantation results in a small hypocellular mass of splenic tissue with a different composition of lymphocyte subsets and does not correct the obvious effect of splenectomy on lymphocyte subpopulations in lymph nodes.
Full text
PDF

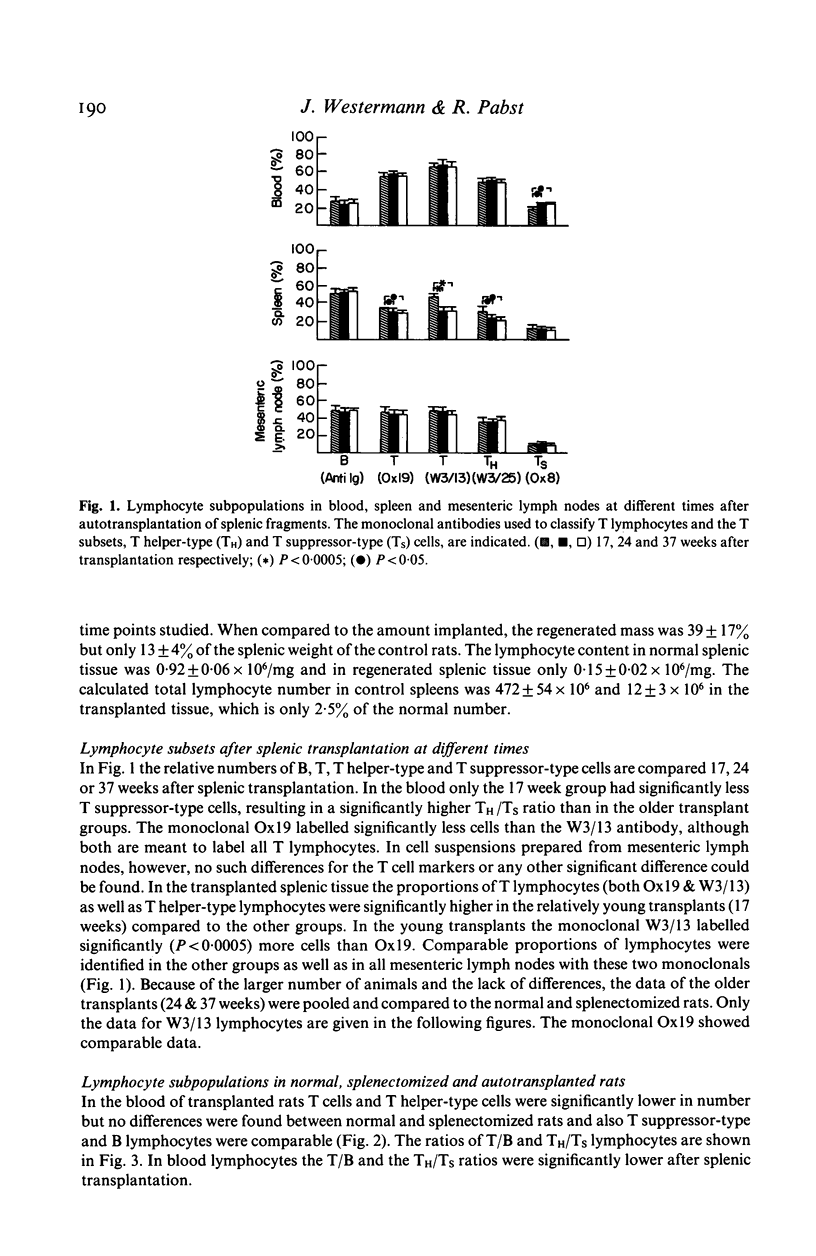
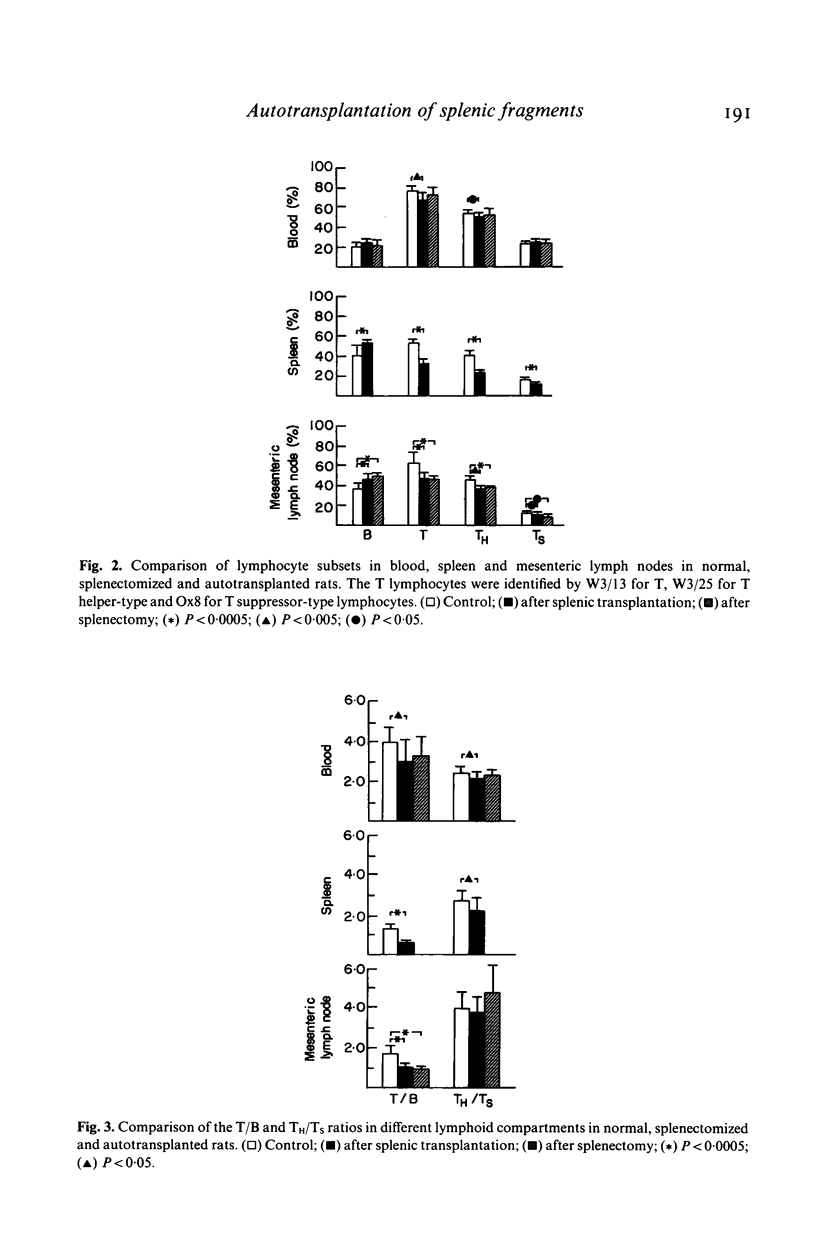



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Binns R. M., Pabst R., Licence S. T. Classification of lymphocytes recirculating in the spleen. Immunology. 1981 Oct;44(2):273–279. [PMC free article] [PubMed] [Google Scholar]
- Bowman C. A., Van Wyck D. B., Witte M. H., Witte C. L. Antibody formation by subcutaneous and omental splenic autotransplants in rats. J Am Med Womens Assoc. 1977 Nov;32(11):409-13, 419. [PubMed] [Google Scholar]
- Cohen R. C., Ferrante A. Immune dysfunction in the presence of residual splenic tissue. Arch Dis Child. 1982 Jul;57(7):523–527. doi: 10.1136/adc.57.7.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper M. J., Williamson R. C. Splenectomy: indications, hazards and alternatives. Br J Surg. 1984 Mar;71(3):173–180. doi: 10.1002/bjs.1800710302. [DOI] [PubMed] [Google Scholar]
- Dickerman J. D., Horner S. R., Coil J. A., Gump D. W. The protective effect of intraperitoneal splenic autotransplants in mice exposed to an aerosolized suspension of type III Streptococcus pneumoniae. Blood. 1979 Aug;54(2):354–358. [PubMed] [Google Scholar]
- Dijkstra C. D., Langevoort H. L. Regeneration of splenic tissue after autologous subcutaneous implantation: development of non-lymphoid cells in the white pulp of the rat spleen. Cell Tissue Res. 1982;222(1):69–79. doi: 10.1007/BF00218289. [DOI] [PubMed] [Google Scholar]
- Drew P. A., Kiroff G. K., Ferrante A., Cohen R. C. Alterations in immunoglobulin synthesis by peripheral blood mononuclear cells from splenectomized patients with and without splenic regrowth. J Immunol. 1984 Jan;132(1):191–196. [PubMed] [Google Scholar]
- Graffner H., Gullstrand P., Hallberg T. Immunocompetence after incidental splenectomy. Scand J Haematol. 1982 May;28(5):369–375. doi: 10.1111/j.1600-0609.1982.tb00541.x. [DOI] [PubMed] [Google Scholar]
- Horton J., Ogden M. E., Williams S., Coln D. The importance of splenic blood flow in clearing pneumococcal organisms. Ann Surg. 1982 Feb;195(2):172–176. doi: 10.1097/00000658-198202000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanng Nielsen J., Tauris P., Johnsen H. E., Ellegaard J. The cellular immune response after splenectomy in humans. Impaired immunoglobulin synthesis in vitro. Scand J Haematol. 1983 Jul;31(1):85–95. doi: 10.1111/j.1600-0609.1983.tb02141.x. [DOI] [PubMed] [Google Scholar]
- Livingston C. D., Levine B. A., Lecklitner M. L., Sirinek K. R. Incidence and function of residual splenic tissue following splenectomy for trauma in adults. Arch Surg. 1983 May;118(5):617–620. doi: 10.1001/archsurg.1983.01390050083016. [DOI] [PubMed] [Google Scholar]
- Malangoni M. A., Dawes L. G., Droege E. A., Rao S. A., Collier B. D., Almagro U. A. Splenic phagocytic function after partial splenectomy and splenic autotransplantation. Arch Surg. 1985 Mar;120(3):275–278. doi: 10.1001/archsurg.1985.01390270015003. [DOI] [PubMed] [Google Scholar]
- Mason D. W., Arthur R. P., Dallman M. J., Green J. R., Spickett G. P., Thomas M. L. Functions of rat T-lymphocyte subsets isolated by means of monoclonal antibodies. Immunol Rev. 1983;74:57–82. doi: 10.1111/j.1600-065x.1983.tb01084.x. [DOI] [PubMed] [Google Scholar]
- Millard R. E., Banerjee D. K. Changes in T and B blood lymphocytes after splenectomy. J Clin Pathol. 1979 Oct;32(10):1045–1049. doi: 10.1136/jcp.32.10.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen J. L., Andersen P., Ellegaard J. Influence of residual splenic tissue on autoantibodies in splenectomized patients. Scand J Haematol. 1982 Apr;28(4):273–277. doi: 10.1111/j.1600-0609.1982.tb00526.x. [DOI] [PubMed] [Google Scholar]
- Pabst R., Hafke R., Hillebrand J. Enhanced regeneration of transplanted splenic tissue by increased work load to the splenic compartments. J Trauma. 1985 Apr;25(4):326–328. doi: 10.1097/00005373-198504000-00008. [DOI] [PubMed] [Google Scholar]
- Pabst R., Hafke R., Hillebrand J. Enhanced regeneration of transplanted splenic tissue by increased work load. Adv Exp Med Biol. 1985;186:565–569. doi: 10.1007/978-1-4613-2463-8_69. [DOI] [PubMed] [Google Scholar]
- Pabst R., Reilmann H. Regeneration of heterotopically transplanted autologous splenic tissue. Cell Tissue Res. 1980;209(1):137–143. doi: 10.1007/BF00219930. [DOI] [PubMed] [Google Scholar]
- Pabst R., Trepel F. The predominant role of the spleen in lymphocyte recirculation. II. Pre- and postsplenectomy retransfusion studies in young pigs. Cell Tissue Kinet. 1976 Mar;9(2):179–189. [PubMed] [Google Scholar]
- Reinecke G., Pabst R. Subsets of blood, spleen and recirculating lymphocytes in man. Clin Exp Immunol. 1983 Sep;53(3):672–678. [PMC free article] [PubMed] [Google Scholar]
- Sekikawa T., Shatney C. H. Septic sequelae after splenectomy for trauma in adults. Am J Surg. 1983 May;145(5):667–673. doi: 10.1016/0002-9610(83)90118-6. [DOI] [PubMed] [Google Scholar]
- Sieber G., Breyer H. G., Herrmann F., Rühl H. Störungen der B-Zellaktivierung bei splenektomierten Patienten. Langenbecks Arch Chir. 1984;363(2):93–101. doi: 10.1007/BF01261058. [DOI] [PubMed] [Google Scholar]
- Sklenar I., Dürig M., Lydick E., Erb P., Harder F. Die Antikörperantwort auf Pneumokokken-Polysaccarid-Antigene nach Splenektomie und Replantation autologen Milzgewebes. Aktuelle Probl Chir Orthop. 1985;30:107–112. [PubMed] [Google Scholar]
- Tauris P., Nielsen J. L. Irradiated autologous T cells restore the in vitro responsiveness of PWM-activated peripheral blood lymphocytes from splenectomized individuals. Acta Pathol Microbiol Immunol Scand C. 1983 Aug;91(4):257–261. [PubMed] [Google Scholar]
- Van Wyck D. B., Witte M. H., Witte C. L., Thies A. C., Jr Critical splenic mass for survival from experimental pneumococcemia. J Surg Res. 1980 Jan;28(1):14–17. doi: 10.1016/0022-4804(80)90076-1. [DOI] [PubMed] [Google Scholar]
- Westerhausen M., Wörsdörfer O., Gessner U., De Giuli R., Senn H. J. Immunological changes following posttraumatic splenectomy. Blut. 1981 Dec;43(6):345–353. doi: 10.1007/BF00320312. [DOI] [PubMed] [Google Scholar]
- Witte M. H., Witte C. L., Van Wyck D. B., Farrell K. J. Preservation of the spleen. Lymphology. 1983 Jun;16(2):128–137. [PubMed] [Google Scholar]


