Abstract
In order to determine the role of different T lymphocyte subsets in the pathogenesis of low-dose streptozotocin (LD-Sz) induced diabetes, we treated mice with Sz together with repeated injections of rat monoclonal antibodies (MoAb) with specificity towards the mouse T cell differentiation markers L3T4 ('helper/inducer' T cells and some macrophages), Lyt-2 ('cytotoxic/suppressor' T cells and NK cells) and Thy-1 (pan T lymphocytes). Treatment depleted target cells in peripheral blood and spleen; decreased the ability of spleen cells to respond to mitogens; and, in the case of depletion of the L3T4 T cell subset, prevented a humoral immune response to SRBC. Treatment with MoAb against either of the two T cell subtypes could protect from hyperglycaemia and loss of body weight, suggesting that both T cell subsets were implicated in the development of LD-Sz induced diabetes. Immunocytochemical analysis of pancreatic sections showed that both L3T4+ and Lyt-2+ cells participated in islet infiltration together with macrophages. Treatment with MoAb markedly reduced islet infiltration by both L3T4+ and Lyt-2+ cells but not by macrophages. The suppressive effect of MoAb against either L3T4 or Lyt-2 on diabetes development suggests that the pathomechanism involved is different from that in experimental autoimmune neuritis and adjuvant arthritis where Lyt-2 cells are not involved.
Full text
PDF

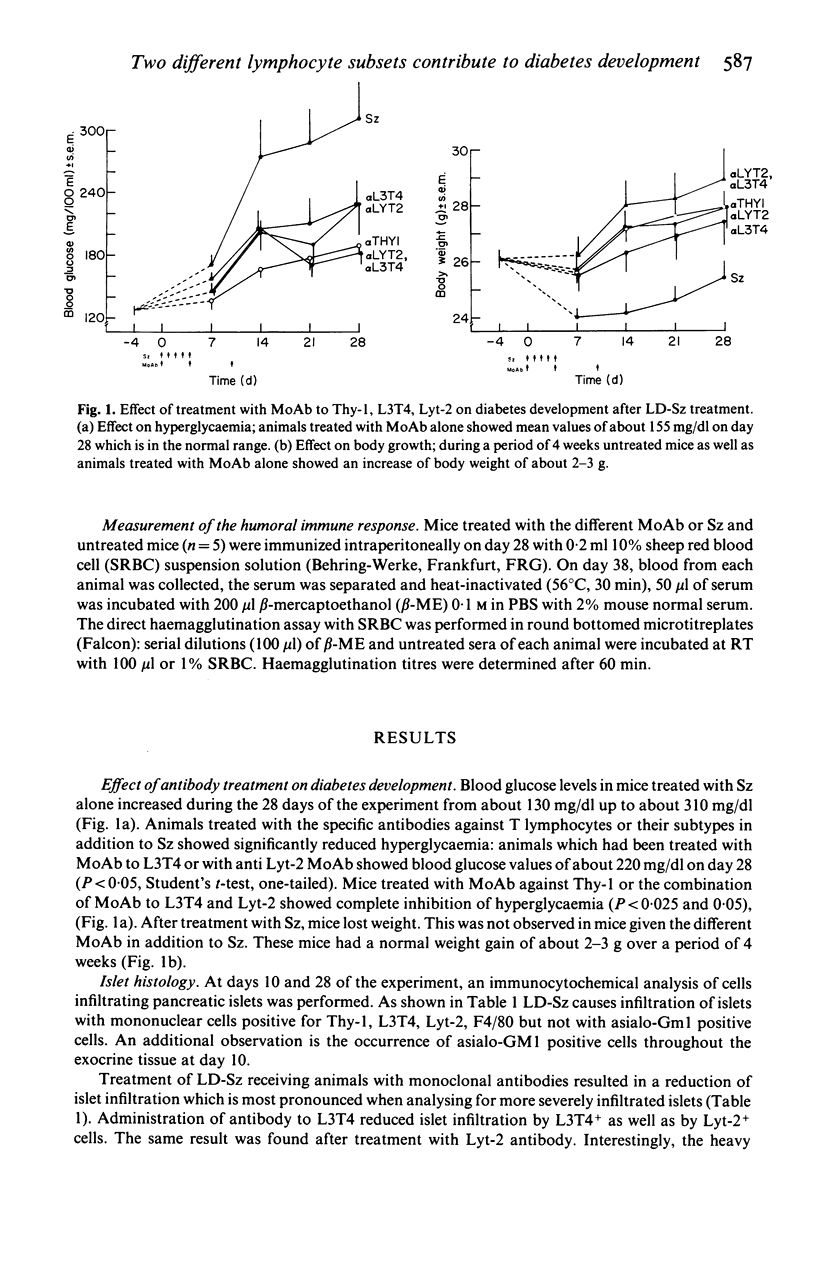
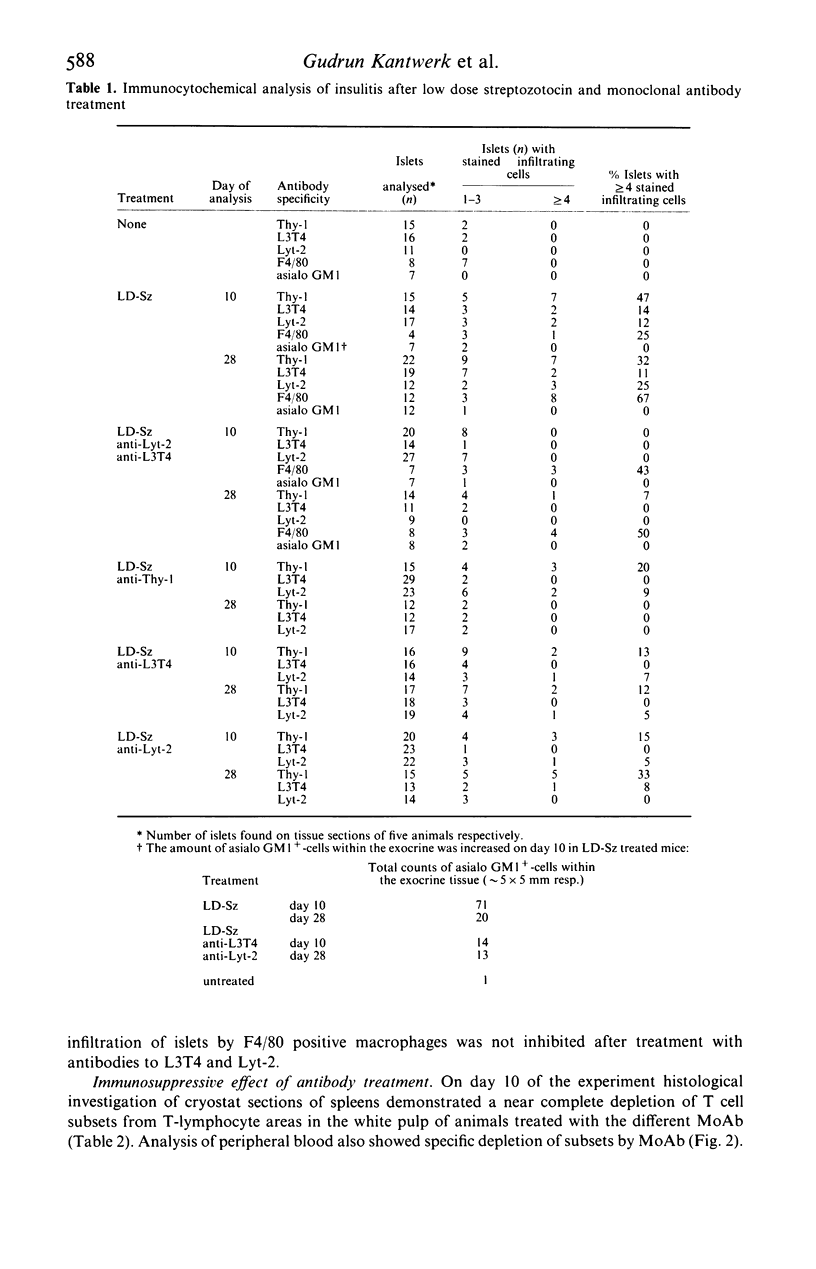
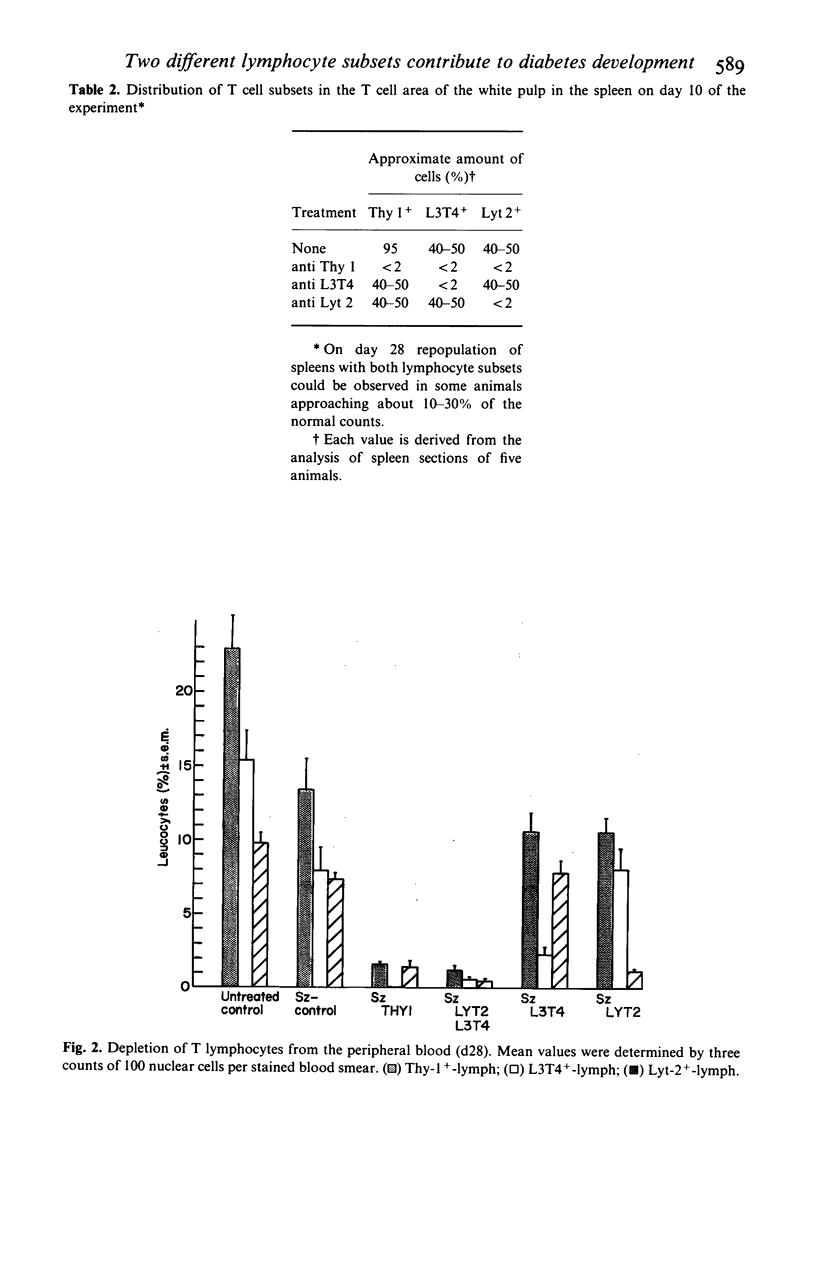
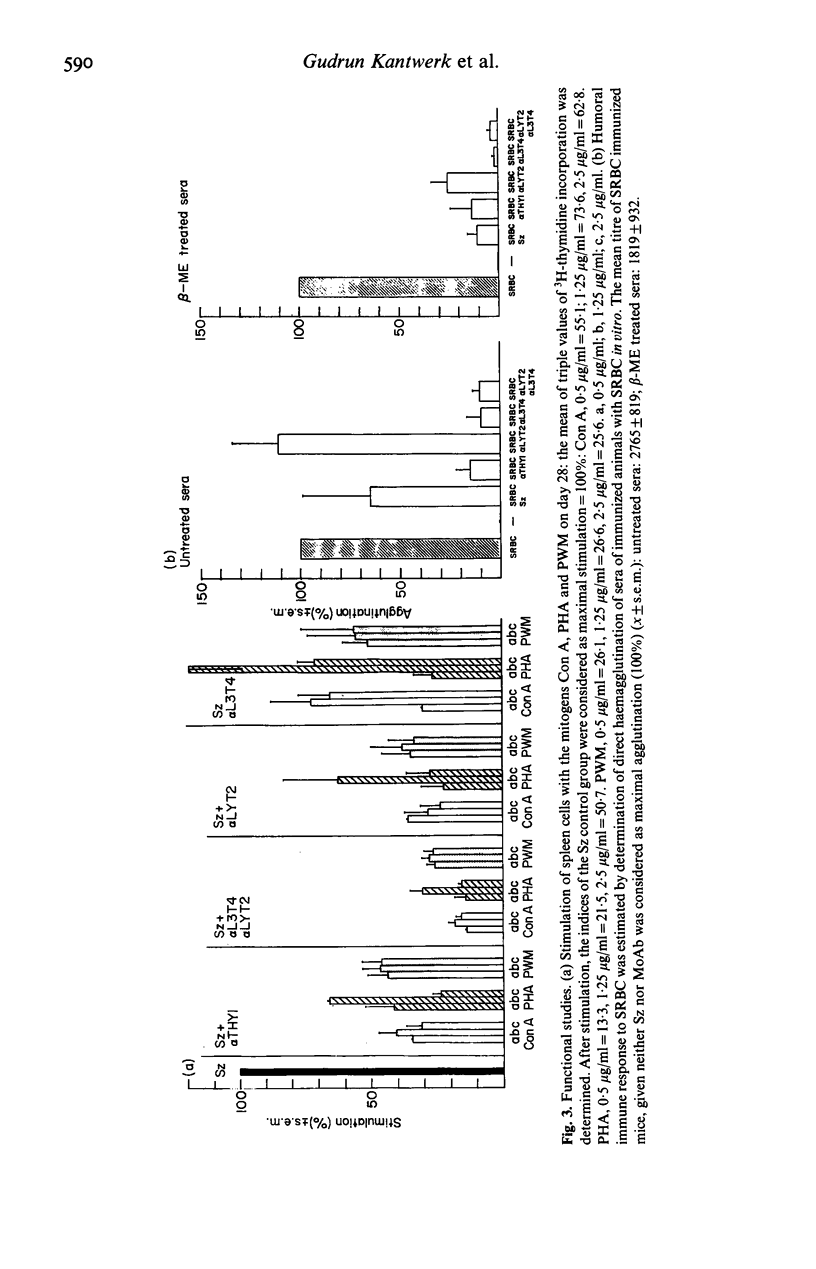


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cobbold S. P., Jayasuriya A., Nash A., Prospero T. D., Waldmann H. Therapy with monoclonal antibodies by elimination of T-cell subsets in vivo. Nature. 1984 Dec 6;312(5994):548–551. doi: 10.1038/312548a0. [DOI] [PubMed] [Google Scholar]
- Cobbold S. P., Martin G., Qin S., Waldmann H. Monoclonal antibodies to promote marrow engraftment and tissue graft tolerance. Nature. 1986 Sep 11;323(6084):164–166. doi: 10.1038/323164a0. [DOI] [PubMed] [Google Scholar]
- Cobbold S., Martin G., Waldmann H. Monoclonal antibodies for the prevention of graft-versus-host disease and marrow graft rejection. The depletion of T cell subsets in vitro and in vivo. Transplantation. 1986 Sep;42(3):239–247. doi: 10.1097/00007890-198609000-00003. [DOI] [PubMed] [Google Scholar]
- Cobbold S., Waldmann H. Skin allograft rejection by L3/T4+ and Lyt-2+ T cell subsets. Transplantation. 1986 May;41(5):634–639. doi: 10.1097/00007890-198605000-00016. [DOI] [PubMed] [Google Scholar]
- Feutren G., Papoz L., Assan R., Vialettes B., Karsenty G., Vexiau P., Du Rostu H., Rodier M., Sirmai J., Lallemand A. Cyclosporin increases the rate and length of remissions in insulin-dependent diabetes of recent onset. Results of a multicentre double-blind trial. Lancet. 1986 Jul 19;2(8499):119–124. doi: 10.1016/s0140-6736(86)91943-4. [DOI] [PubMed] [Google Scholar]
- Galfrè G., Milstein C., Wright B. Rat x rat hybrid myelomas and a monoclonal anti-Fd portion of mouse IgG. Nature. 1979 Jan 11;277(5692):131–133. doi: 10.1038/277131a0. [DOI] [PubMed] [Google Scholar]
- Gepts W., Lecompte P. M. The pancreatic islets in diabetes. Am J Med. 1981 Jan;70(1):105–115. doi: 10.1016/0002-9343(81)90417-4. [DOI] [PubMed] [Google Scholar]
- Guimezanes A., Schmitt-Verhulst A. M. Inhibition of helper function with anti-Lyt-2 or anti-L3T4 monoclonal antibodies depending on stimulating antigens. Eur J Immunol. 1985 Dec;15(12):1187–1191. doi: 10.1002/eji.1830151209. [DOI] [PubMed] [Google Scholar]
- Holmdahl R., Olsson T., Moran T., Klareskog L. In vivo treatment of rats with monoclonal anti-T-cell antibodies. Immunohistochemical and functional analysis in normal rats and in experimental allergic neuritis. Scand J Immunol. 1985 Aug;22(2):157–169. doi: 10.1111/j.1365-3083.1985.tb01868.x. [DOI] [PubMed] [Google Scholar]
- Jefferies W. A., Green J. R., Williams A. F. Authentic T helper CD4 (W3/25) antigen on rat peritoneal macrophages. J Exp Med. 1985 Jul 1;162(1):117–127. doi: 10.1084/jem.162.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolb H., Ben-Nun A., Cohen I. R., Barberena I., Kiesel U. Autoimmune T-lymphocytes with specificity for pancreatic islet antigens. Immunol Lett. 1985;9(1):29–32. doi: 10.1016/0165-2478(85)90090-2. [DOI] [PubMed] [Google Scholar]
- Kolb H., Kiesel U., Flohr K., Greulich B., Freytag G., Kolb-Bachofen V. Cell mediated immunity to islet cells: lessons from animal studies. Behring Inst Mitt. 1984 Jul;(75):33–38. [PubMed] [Google Scholar]
- Larsson P., Holmdahl R., Dencker L., Klareskog L. In vivo treatment with W3/13 (anti-pan T) but not with OX8 (anti-suppressor/cytotoxic T) monoclonal antibodies impedes the development of adjuvant arthritis in rats. Immunology. 1985 Nov;56(3):383–391. [PMC free article] [PubMed] [Google Scholar]
- Oschilewski M., Schwab E., Kiesel U., Opitz U., Stünkel K., Kolb-Bachofen V., Kolb H. Administration of silica or monoclonal antibody to Thy-1 prevents low-dose streptozotocin-induced diabetes in mice. Immunol Lett. 1986 Jun;12(5-6):289–294. doi: 10.1016/0165-2478(86)90032-5. [DOI] [PubMed] [Google Scholar]
- RAKIETEN N., RAKIETEN M. L., NADKARNI M. R. Studies on the diabetogenic action of streptozotocin (NSC-37917). Cancer Chemother Rep. 1963 May;29:91–98. [PubMed] [Google Scholar]
- Rossini A. A., Williams R. M., Appel M. C., Like A. A. Complete protection from low-dose streptozotocin-induced diabetes in mice. Nature. 1978 Nov 9;276(5684):182–184. doi: 10.1038/276182a0. [DOI] [PubMed] [Google Scholar]
- Sprent J., Schaefer M. Capacity of purified Lyt-2+ T cells to mount primary proliferative and cytotoxic responses to Ia- tumour cells. Nature. 1986 Aug 7;322(6079):541–544. doi: 10.1038/322541a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun D., Wekerle H. Ia-restricted encephalitogenic T lymphocytes mediating EAE lyse autoantigen-presenting astrocytes. Nature. 1986 Mar 6;320(6057):70–72. doi: 10.1038/320070a0. [DOI] [PubMed] [Google Scholar]


