Abstract
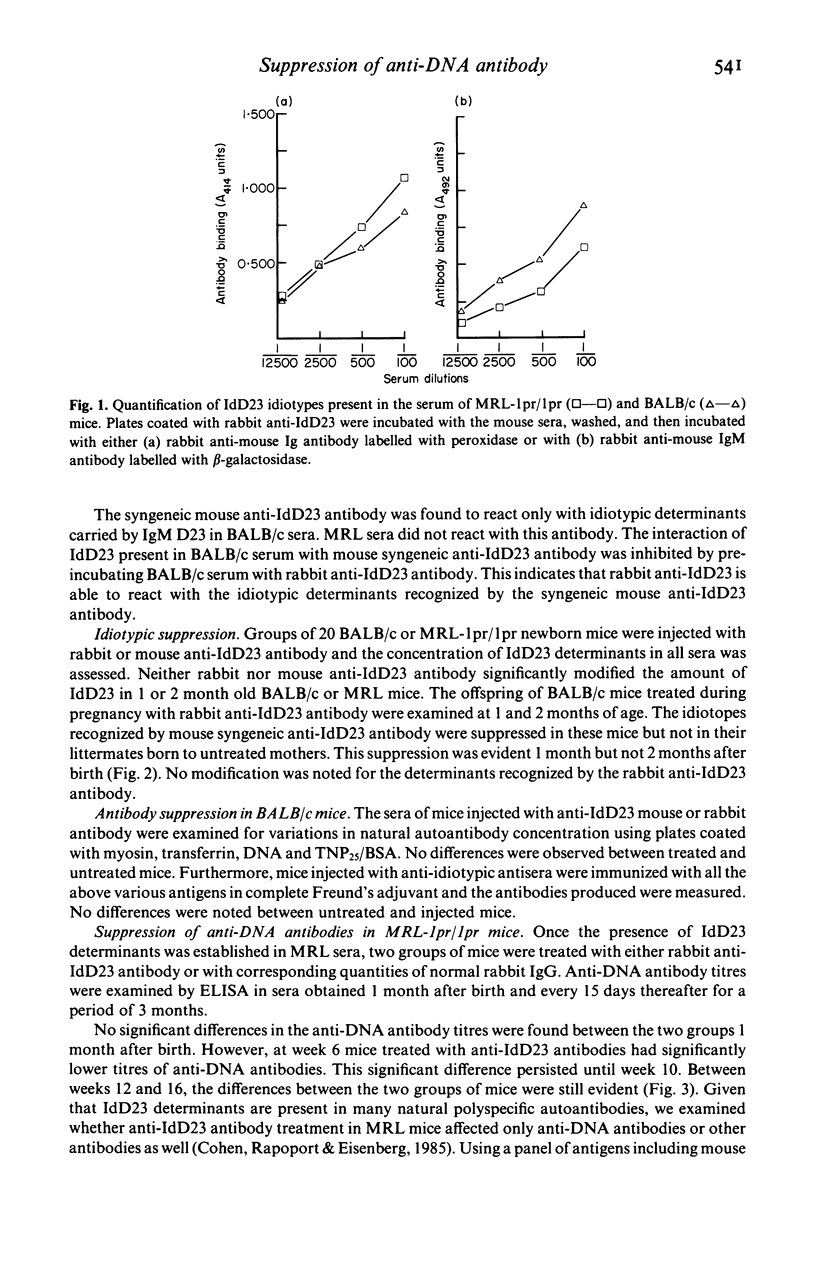
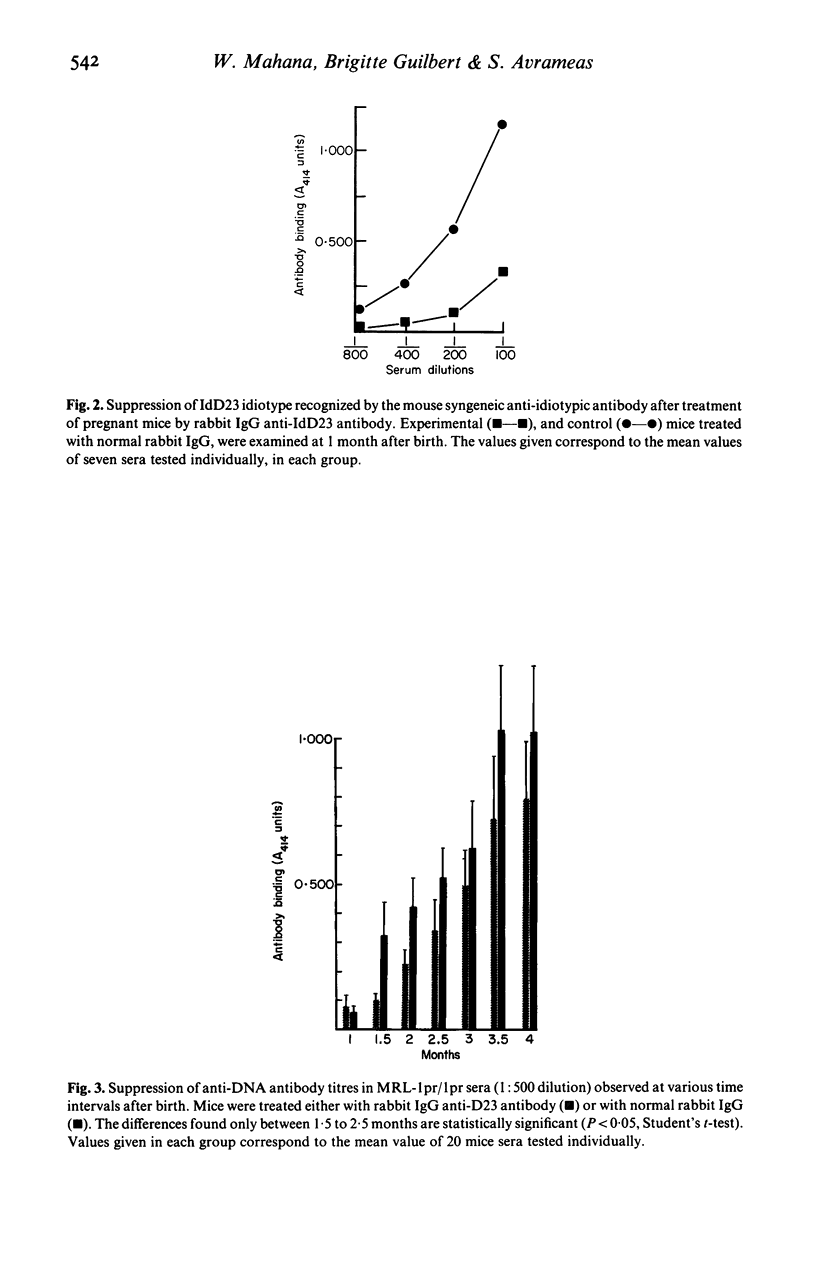
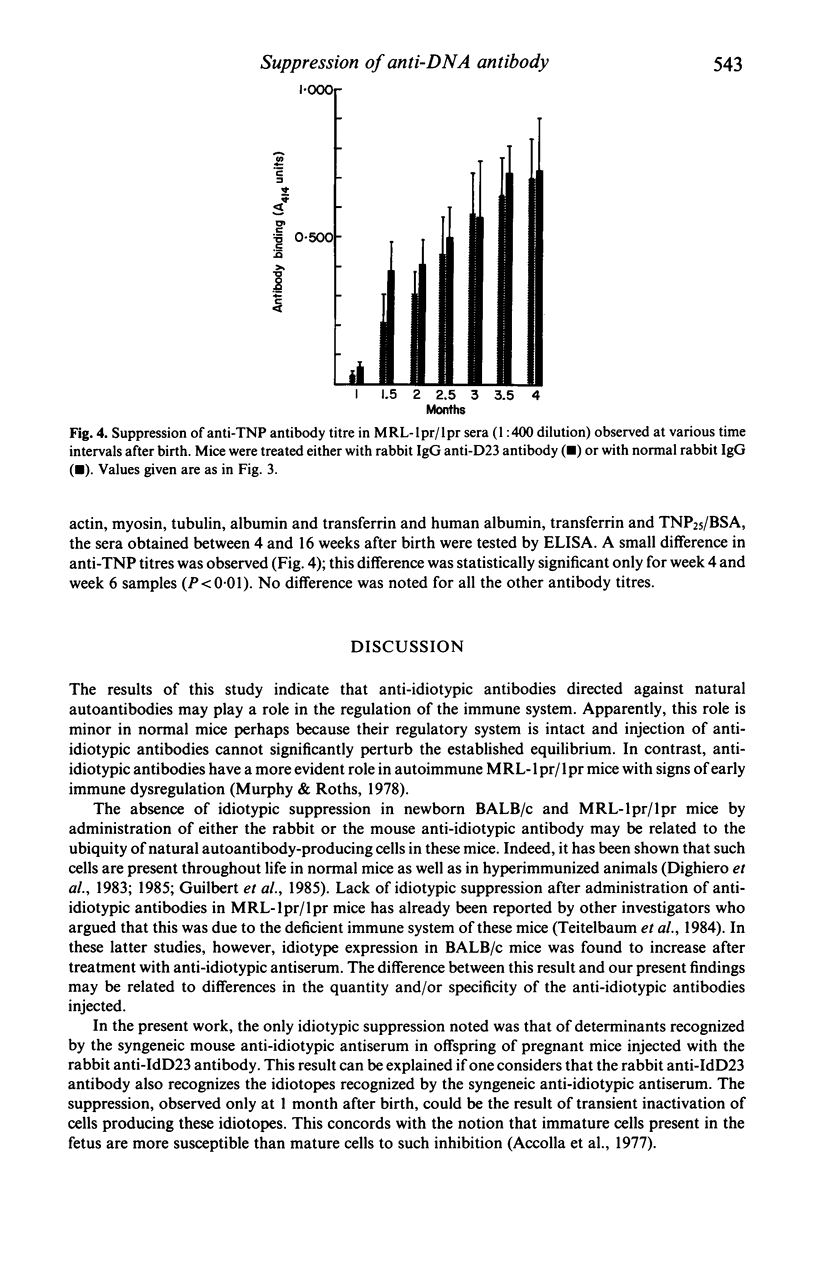
Antibodies against idiotypic determinants carried by a monoclonal polyspecific natural autoantibody were raised in rabbits and in syngeneic BALB/c mice. These anti-idiotypic antibodies were administered to newborn and to pregnant BALB/c mice and to MRL-lpr/lpr mice. Serial measurements of the idiotypes, naturally occurring autoantibodies, and antibodies obtained after antigenic stimulation were performed in the sera of the injected mice and in the offspring of pregnant mice. No idiotypic suppression was noted in newborn injected mice. Transient suppression of idiotypes recognized by the syngeneic anti-idiotypic antibody was noted in the offspring of pregnant mice injected with the rabbit polyclonal anti-idiotypic antiserum. No changes in naturally occurring autoantibodies or in antibodies appearing after antigenic stimulation were noted in BALB/c mice. In contrast, a significant decrease of spontaneously occurring anti-DNA antibodies was found in MRL-lpr/lpr mice treated with rabbit polyclonal anti-idiotypic antiserum. Furthermore in these mice a slight decrease of anti-TNP antibodies was also observed. These results suggest that anti-idiotypic antibodies directed against natural autoantibodies may play a regulatory role in the immune system; this role is more easily appreciated in mice suffering from immune dysregulation.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdou N. I., Wall H., Lindsley H. B., Halsey J. F., Suzuki T. Network theory in autoimmunity. In vitro suppression of serum anti-DNA antibody binding to DNA by anti-idiotypic antibody in systemic lupus erythematosus. J Clin Invest. 1981 May;67(5):1297–1304. doi: 10.1172/JCI110158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Accolla R. S., Gearhart P. J., Sigal N. H., Cancro M. P., Klinman N. R. Idiotype-specific neonatal suppression of phosphorylcholine-responsive B cells. Eur J Immunol. 1977 Dec;7(12):876–881. doi: 10.1002/eji.1830071211. [DOI] [PubMed] [Google Scholar]
- Avrameas S., Guilbert B., Dighiero G. Natural antibodies against tubulin, actin myoglobin, thyroglobulin, fetuin, albumin and transferrin are present in normal human sera, and monoclonal immunoglobulins from multiple myeloma and Waldenström's macroglobulinemia may express similar antibody specificities. Ann Immunol (Paris) 1981 Mar-Apr;132C(2):231–236. doi: 10.1016/0769-2625(81)90031-3. [DOI] [PubMed] [Google Scholar]
- Brown C. A., Carey K., Colvin R. B. Inhibition of autoimmune tubulointerstitial nephritis in guinea pigs by heterologous antisera containing anti-idiotype antibodies. J Immunol. 1979 Nov;123(5):2102–2107. [PubMed] [Google Scholar]
- Cohen P. L., Rapoport R. G., Eisenberg R. A. Hidden autoantibodies against common serum proteins in murine systemic lupus erythematosus. Detection by in vitro plaque-forming cell assay. J Exp Med. 1985 Jun 1;161(6):1587–1592. doi: 10.1084/jem.161.6.1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dighiero G., Guilbert B., Avrameas S. Naturally occurring antibodies against nine common antigens in humans sera. II. High incidence of monoclonal Ig exhibiting antibody activity against actin and tubulin and sharing antibody specificities with natural antibodies. J Immunol. 1982 Jun;128(6):2788–2792. [PubMed] [Google Scholar]
- Dighiero G., Lymberi P., Holmberg D., Lundquist I., Coutinho A., Avrameas S. High frequency of natural autoantibodies in normal newborn mice. J Immunol. 1985 Feb;134(2):765–771. [PubMed] [Google Scholar]
- Dighiero G., Lymberi P., Mazié J. C., Rouyre S., Butler-Browne G. S., Whalen R. G., Avrameas S. Murine hybridomas secreting natural monoclonal antibodies reacting with self antigens. J Immunol. 1983 Nov;131(5):2267–2272. [PubMed] [Google Scholar]
- Dwyer D. S., Bradley R. J., Urquhart C. K., Kearney J. F. Naturally occurring anti-idiotypic antibodies in myasthenia gravis patients. Nature. 1983 Feb 17;301(5901):611–614. doi: 10.1038/301611a0. [DOI] [PubMed] [Google Scholar]
- Eichmann K. Idiotype suppression. I. Influence of the dose and of the effector functions of anti-idiotypic antibody on the production of an idiotype. Eur J Immunol. 1974 Apr;4(4):296–302. doi: 10.1002/eji.1830040413. [DOI] [PubMed] [Google Scholar]
- Ey P. L., Prowse S. J., Jenkin C. R. Isolation of pure IgG1, IgG2a and IgG2b immunoglobulins from mouse serum using protein A-sepharose. Immunochemistry. 1978 Jul;15(7):429–436. doi: 10.1016/0161-5890(78)90070-6. [DOI] [PubMed] [Google Scholar]
- Fung J., Köhler H. Mechanism of neonatal idiotype suppression. I. State of the suppressed B cells. J Immunol. 1980 Nov;125(5):1998–2003. [PubMed] [Google Scholar]
- Fung J., Köhler H. Mechanism of neonatal idiotype suppression. II. Alterations in the T cell compartment suppress the maturation of B cell precursors. J Immunol. 1980 Dec;125(6):2489–2495. [PubMed] [Google Scholar]
- Guilbert B., Dighiero G., Avrameas S. Naturally occurring antibodies against nine common antigens in human sera. I. Detection, isolation and characterization. J Immunol. 1982 Jun;128(6):2779–2787. [PubMed] [Google Scholar]
- Guilbert B., Mahana W., Gilbert M., Mazie J. C., Avrameas S. Presence of natural autoantibodies in hyperimmunized mice. Immunology. 1985 Nov;56(3):401–408. [PMC free article] [PubMed] [Google Scholar]
- Hahn B. H., Ebling F. M. Suppression of NZB/NZW murine nephritis by administration of a syngeneic monoclonal antibody to DNA. Possible role of anti-idiotypic antibodies. J Clin Invest. 1983 Jun;71(6):1728–1736. doi: 10.1172/JCI110927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmberg D., Forsgren S., Ivars F., Coutinho A. Reactions among IgM antibodies derived from normal, neonatal mice. Eur J Immunol. 1984 May;14(5):435–441. doi: 10.1002/eji.1830140510. [DOI] [PubMed] [Google Scholar]
- Jacob L., Tron F. Induction of anti-DNA autoanti-idiotypic antibodies in (NZB X NZW)F1 mice: possible role for specific immune suppression. Clin Exp Immunol. 1984 Nov;58(2):293–299. [PMC free article] [PubMed] [Google Scholar]
- Jerne N. K. Towards a network theory of the immune system. Ann Immunol (Paris) 1974 Jan;125C(1-2):373–389. [PubMed] [Google Scholar]
- Karsenti E., Guilbert B., Bornens M., Avrameas S. Anticorps anti-actine et anti-tubuline: leur présence dans les sérums d'animaux non immunisés. Ann Immunol (Paris) 1977 Jan-Mar;128(1-2):195–200. [PubMed] [Google Scholar]
- Kim B. S., Hopkins W. J. Tolerance rendered by neonatal treatment with anti-idiotypic antibodies: induction and maintenance in athymic mice. Cell Immunol. 1978 Feb;35(2):460–465. doi: 10.1016/0008-8749(78)90164-8. [DOI] [PubMed] [Google Scholar]
- Little J. R., Eisen H. N. Preparation and characterization of antibodies specific for the 2,4,6-trinitrophenyl group. Biochemistry. 1966 Nov;5(11):3385–3395. doi: 10.1021/bi00875a001. [DOI] [PubMed] [Google Scholar]
- Lymberi P., Dighiero G., Ternynck T., Avrameas S. A high incidence of cross-reactive idiotypes among murine natural autoantibodies. Eur J Immunol. 1985 Jul;15(7):702–707. doi: 10.1002/eji.1830150712. [DOI] [PubMed] [Google Scholar]
- Matsiota P., Druet P., Dosquet P., Guilbert B., Avrameas S. Natural autoantibodies in systemic lupus erythematosus. Clin Exp Immunol. 1987 Jul;69(1):79–88. [PMC free article] [PubMed] [Google Scholar]
- PLESCIA O. J., BRAUN W., PALCZUK N. C. PRODUCTION OF ANTIBODIES TO DENATURED DEOXYRIBONUCLEIC ACID (DNA). Proc Natl Acad Sci U S A. 1964 Aug;52:279–285. doi: 10.1073/pnas.52.2.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serban D., Rordorf-Adam C., Sun Y. Z., Gordon J. Murine polyspecific antibodies. I. Monoclonal and serum anti-DNA antibodies cross-reactive with 2,4,6-trinitrophenyl derivatives. J Immunol. 1985 Nov;135(5):3122–3127. [PubMed] [Google Scholar]
- Shelanski M. L., Gaskin F., Cantor C. R. Microtubule assembly in the absence of added nucleotides. Proc Natl Acad Sci U S A. 1973 Mar;70(3):765–768. doi: 10.1073/pnas.70.3.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spudich J. A., Watt S. The regulation of rabbit skeletal muscle contraction. I. Biochemical studies of the interaction of the tropomyosin-troponin complex with actin and the proteolytic fragments of myosin. J Biol Chem. 1971 Aug 10;246(15):4866–4871. [PubMed] [Google Scholar]
- Teitelbaum D., Rauch J., Stollar B. D., Schwartz R. S. In vivo effects of antibodies against a high frequency idiotype of anti-DNA antibodies in MRL mice. J Immunol. 1984 Mar;132(3):1282–1285. [PubMed] [Google Scholar]
- Ternynck T., Avrameas S. A new method using p-benzoquinone for coupling antigens and antibodies to marker substances. Ann Immunol (Paris) 1976 Mar-Apr;127(2):197–208. [PubMed] [Google Scholar]
- Whalen R. G., Butler-Browne G. S., Gros F. Identification of a novel form of myosin light chain present in embryonic muscle tissue and cultured muscle cells. J Mol Biol. 1978 Dec 15;126(3):415–431. doi: 10.1016/0022-2836(78)90049-9. [DOI] [PubMed] [Google Scholar]
- Zanetti M., Bigazzi P. E. Anti-idiotypic immunity and autoimmunity. I. In vitro and in vivo effects of anti-idiotypic antibodies to spontaneously occurring autoantibodies to rat thyroglobulin. Eur J Immunol. 1981 Mar;11(3):187–195. doi: 10.1002/eji.1830110306. [DOI] [PubMed] [Google Scholar]


