Abstract
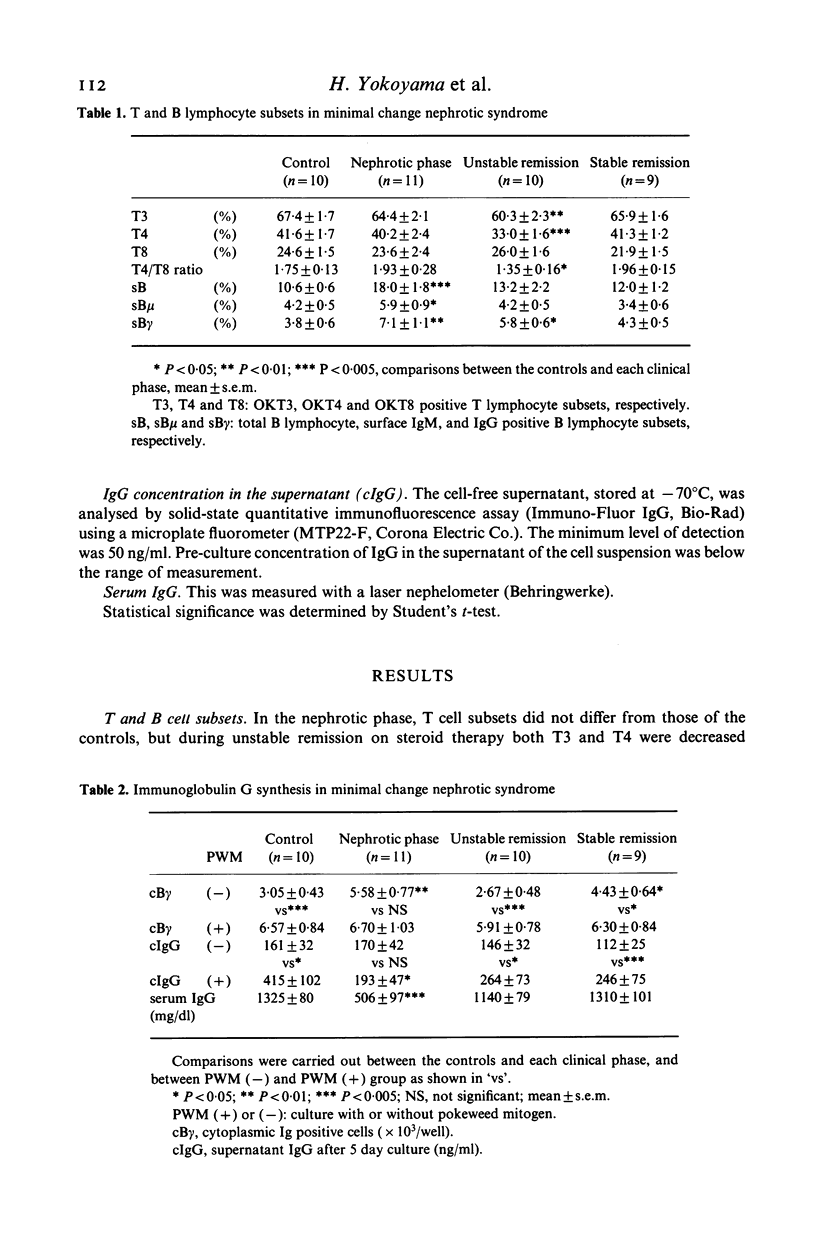
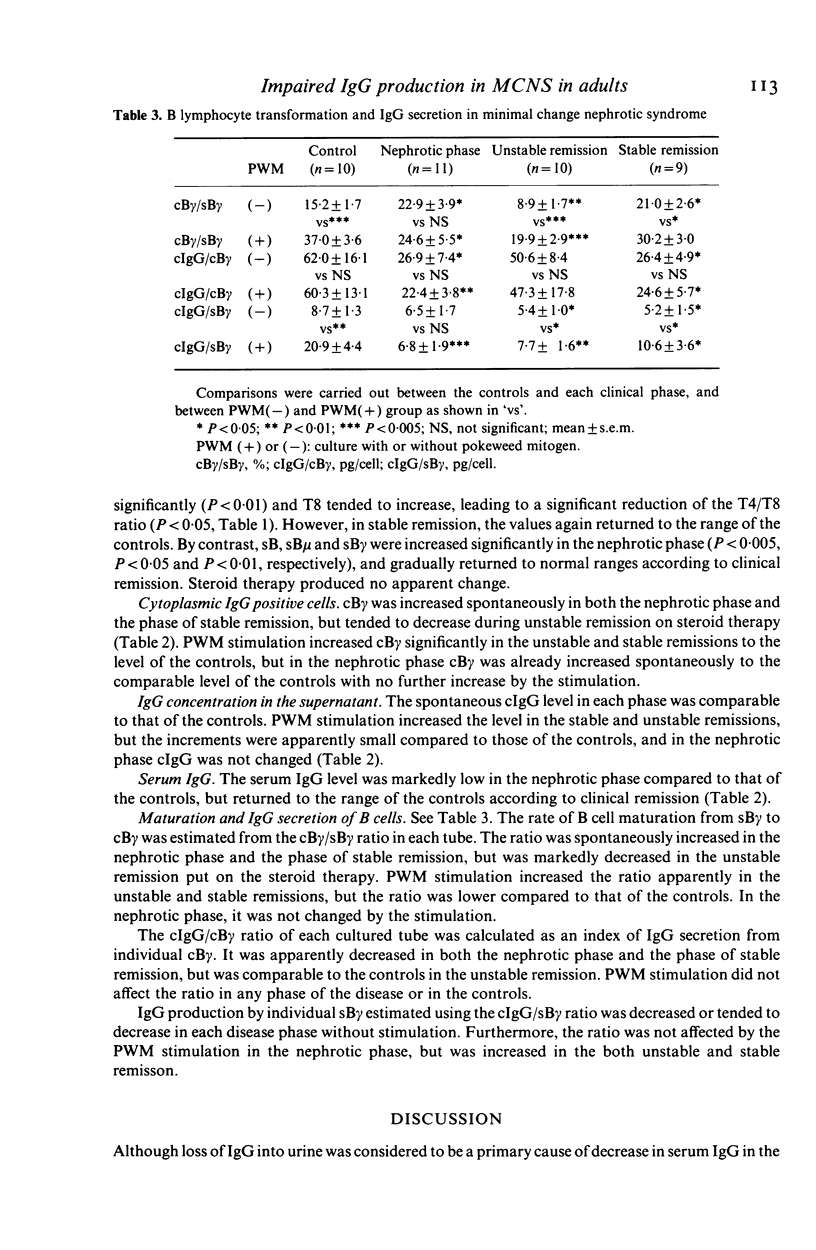
Serum IgG, T and B cell subsets, cytoplasmic IgG positive cells (cB gamma) and IgG in the medium (cIgG) of a 5 day culture of peripheral lymphocytes in both stimulated and non-stimulated (spontaneous) conditions with pokeweed mitogen (PWM) were studied in 30 adult patients with minimal change nephrotic syndrome (MCNS). In the nephrotic phase (11 patients), surface IgG positive B cells (sB gamma) and spontaneous cB gamma increased (P less than 0.05), whereas PWM-stimulated cIgG did not increase, and serum IgG decreased significantly (P less than 0.05). The cB gamma/sB gamma ratio calculated as an index of IgG synthesis in B cells increased spontaneously (P less than 0.05), but did not increase under PWM-stimulation. The cIgG/cB gamma ratio as an index of IgG secretion from each matured B cell, reduced in both spontaneous and stimulated conditions (P less than 0.05, P less than 0.01, respectively). In the phase of unstable remission maintained by steroid therapy (10 patients), these parameters tended to normalize and the OKT4/OKT8 ratio decreased (P less than 0.05), while the ratio remained unchanged in the nephrotic phase. However, after discontinuation of steroid (nine patients), spontaneous cB gamma and the spontaneous cB gamma/sB gamma ratio were again increased, and the cIgG/cB gamma ratio decreased (P less than 0.05) as observed in the nephrotic phase. These results suggest that B cells in patients with MCNS both in the nephrotic state and stable remission after discontinuation of steroid are activated spontaneously, but the secretory process of IgG from the matured cells is impaired, and that steroid improves these abnormalities.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen S. B., Jarnum S., Jensen H., Rossing N. Metabolism of gamma-G-globulin in the nephrotic syndrome in adults. Scand J Clin Lab Invest. 1968;21(1):42–48. doi: 10.3109/00365516809076975. [DOI] [PubMed] [Google Scholar]
- Beale M. G., Nash G. S., Bertovich M. J., MacDermott R. P. Immunoglobulin synthesis by peripheral blood mononuclear cells in minimal change nephrotic syndrome. Kidney Int. 1983 Feb;23(2):380–386. doi: 10.1038/ki.1983.30. [DOI] [PubMed] [Google Scholar]
- Dall'Aglio P., Meroni P. L., Barcellini W., Brigati C., Chizzolini C., De Bartolo G., Migone L., Zanussi C. Altered expression of B lymphocyte surface immunoglobulins in minimal change nephrotic syndrome and focal glomerulosclerosis. Nephron. 1984;37(4):224–228. doi: 10.1159/000183253. [DOI] [PubMed] [Google Scholar]
- Feehally J., Beattie T. J., Brenchley P. E., Coupes B. M., Houston I. B., Mallick N. P., Postlethwaite R. J. Modulation of cellular immune function by cyclophosphamide in children with minimal-change nephropathy. N Engl J Med. 1984 Feb 16;310(7):415–420. doi: 10.1056/NEJM198402163100702. [DOI] [PubMed] [Google Scholar]
- GITLIN D., JANEWAY C. A., FARR L. E. Studies on the metabolism of plasma proteins in the nephrotic syndrome. I. Albumin, gamma-globulin and iron-binding globulin. J Clin Invest. 1956 Jan;35(1):44–56. doi: 10.1172/JCI103251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giangiacomo J., Cleary T. G., Cole B. R., Hoffsten P., Robson A. M. Serum immunoglobulins in the nephrotic syndrome. A possible cause of minimal-change nephrotic syndrome. N Engl J Med. 1975 Jul 3;293(1):8–12. doi: 10.1056/NEJM197507032930103. [DOI] [PubMed] [Google Scholar]
- Herrod H. G., Stapleton F. B., Trouy R. L., Roy S. Evaluation of T lymphocyte subpopulations in children with nephrotic syndrome. Clin Exp Immunol. 1983 Jun;52(3):581–585. [PMC free article] [PubMed] [Google Scholar]
- Heslan J. M., Lautie J. P., Intrator L., Blanc C., Lagrue G., Sobel A. T. Impaired IgG synthesis in patients with the nephrotic syndrome. Clin Nephrol. 1982 Sep;18(3):144–147. [PubMed] [Google Scholar]
- Kearney J. F., Lawton A. R. B lymphocyte differentiation induced by lipopolysaccharide. I. Generation of cells synthesizing four major immunoglobulin classes. J Immunol. 1975 Sep;115(3):671–676. [PubMed] [Google Scholar]
- Kumagai K., Abo T., Sekizawa T., Sasaki M. Studies of surface immunoglobulins on human B lymphocytes. I. Dissociation of cell-bound immunoglobulins with acid pH or at 37 degrees C. J Immunol. 1975 Oct;115(4):982–987. [PubMed] [Google Scholar]
- Melchers F., Andersson J. Factors controlling the B-cell cycle. Annu Rev Immunol. 1986;4:13–36. doi: 10.1146/annurev.iy.04.040186.000305. [DOI] [PubMed] [Google Scholar]
- Reinherz E. L., Kung P. C., Goldstein G., Schlossman S. F. A monoclonal antibody reactive with the human cytotoxic/suppressor T cell subset previously defined by a heteroantiserum termed TH2. J Immunol. 1980 Mar;124(3):1301–1307. [PubMed] [Google Scholar]
- Reinherz E. L., Kung P. C., Goldstein G., Schlossman S. F. A monoclonal antibody with selective reactivity with functionally mature human thymocytes and all peripheral human T cells. J Immunol. 1979 Sep;123(3):1312–1317. [PubMed] [Google Scholar]
- Reinherz E. L., Kung P. C., Goldstein G., Schlossman S. F. Further characterization of the human inducer T cell subset defined by monoclonal antibody. J Immunol. 1979 Dec;123(6):2894–2896. [PubMed] [Google Scholar]
- Shakib F., Hardwicke J., Stanworth D. R., White R. H. Asymmetric depression in the serum level of IgG subclasses in patients with nephrotic syndrome. Clin Exp Immunol. 1977 Jun;28(3):506–511. [PMC free article] [PubMed] [Google Scholar]
- Tani Y., Kida H., Abe T., Tomosugi N., Saito Y., Asamoto T., Hattori N. B lymphocyte subset patterns and their significance in idiopathic glomerulonephritis. Clin Exp Immunol. 1982 Apr;48(1):201–204. [PMC free article] [PubMed] [Google Scholar]
- Yokoyama H., Kida H., Tani Y., Abe T., Tomosugi N., Koshino Y., Hattori N. Immunodynamics of minimal change nephrotic syndrome in adults T and B lymphocyte subsets and serum immunoglobulin levels. Clin Exp Immunol. 1985 Sep;61(3):601–607. [PMC free article] [PubMed] [Google Scholar]


