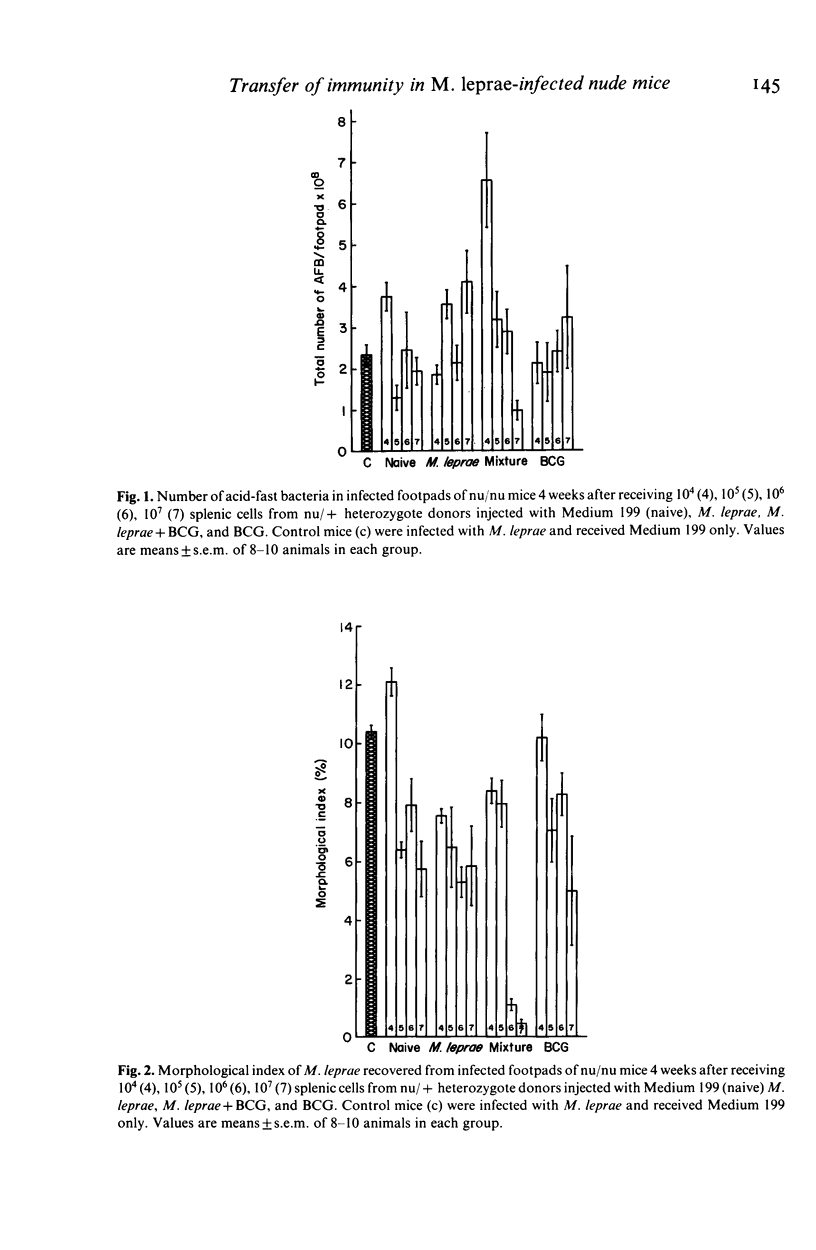
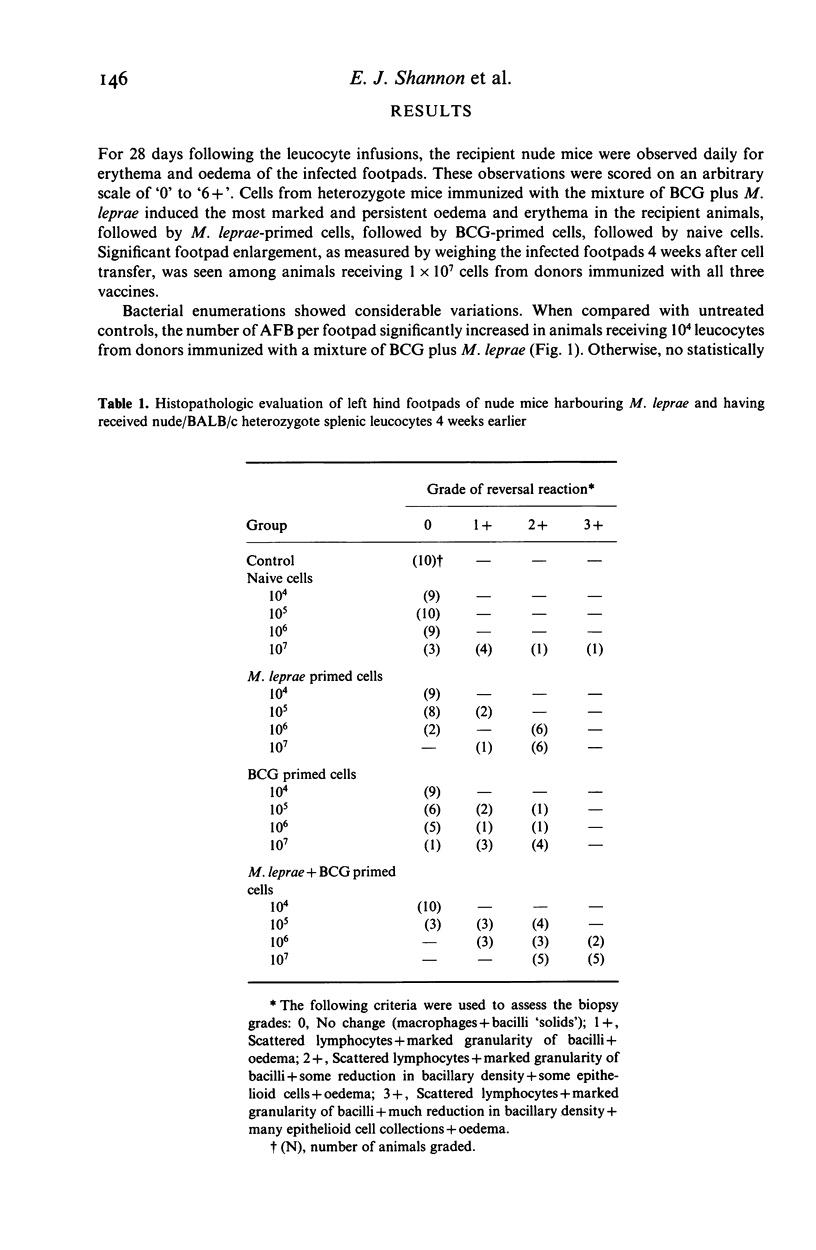
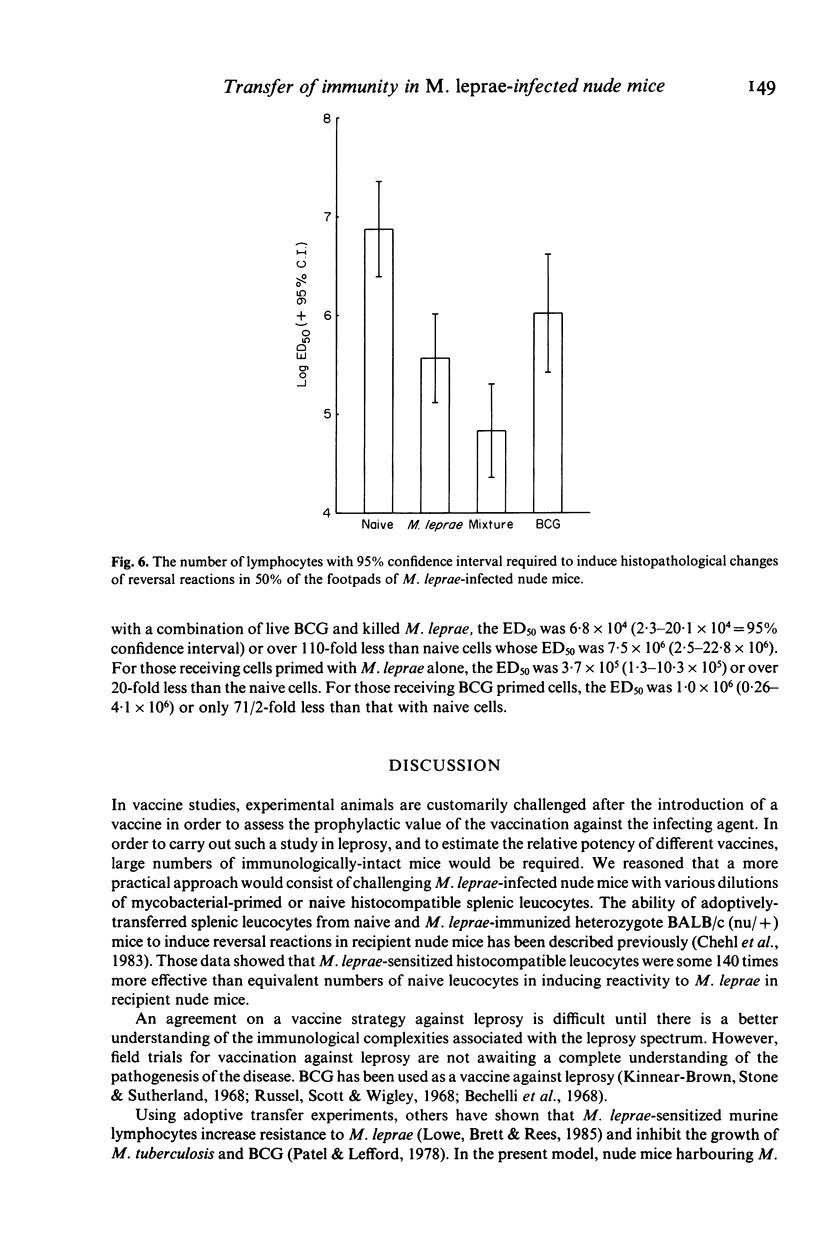
Abstract
Reversal reactions are manifestations of delayed hypersensitivity to M. leprae and are thought to be usually accompanied by manifestations of effective cell-mediated immunity (CMI) as measured by bacterial clearing. These experiments were designed to study the induction of reversal reactions in M. leprae-infected, congenitally athymic nude mice using adoptive transfer of CMI. Splenic cell suspensions derived from unimmunized heterozygous nu/+ mice, and those vaccinated with heat-killed M. leprae, viable BCG and a mixture of the two antigens were diluted to contain 10(4), 10(5), 10(6), 10(7) lymphocytes/0.1 ml and infused intravenously into multibacillary nude mice. The production of reversal reactions in leprous nude mice in response to adoptively transferred CMI was studied in a quantitative fashion. Dose responsive induction of reversal reactions, apparent by footpad inflammation and swelling, decreased morphological indices (MI) of the bacteria and mononuclear cell infiltrations, histopathologically, were observed. For nude mice receiving cells primed with 3.9 X 10(5) living BCG alone, the effective dose 50% (ED50) was 1.0 x 10(6) lymphocytes to induce reversal reactions. For those receiving cells primed with 10(7) M. leprae the ED50 was 3.7 x 10(5) lymphocytes. For nude mice receiving cells primed with a mixture consisting of 1/2 the above dose of BCG + 1/2 the above dose of M. leprae, the ED50 was 6.8 x 10(4) lymphocytes.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chehl S., Ruby J., Job C. K., Hastings R. C. The growth of Mycobacterium leprae in nude mice. Lepr Rev. 1983 Dec;54(4):283–304. doi: 10.5935/0305-7518.19830035. [DOI] [PubMed] [Google Scholar]
- Convit J., Aranzazu N., Pinardi M., Ulrich M. Immunological changes observed in indeterminate and lepromatous leprosy patients and Mitsuda-negative contacts after the inoculation of a mixture of Mycobacterium leprae and BCG. Clin Exp Immunol. 1979 May;36(2):214–220. [PMC free article] [PubMed] [Google Scholar]
- Convit J., Pinardi M. E., Rodríguez Ochoa G., Ulrich M., Avila J. L., Goihman M. Elimination of Mycobacterium leprae subsequent to local in vivo activation of macrophages in lepromatous leprosy by other mycobacteria. Clin Exp Immunol. 1974 Jun;17(2):261–265. [PMC free article] [PubMed] [Google Scholar]
- Convit J., Ulrich M., Aranzazu N. Vaccination in leprosy--observations and interpretations. Int J Lepr Other Mycobact Dis. 1980 Mar;48(1):62–65. [PubMed] [Google Scholar]
- FERNANDEZ J. M., HANKS J. H. Enhancement of resistance to murine leprosy by BCG plus specific antigen. Int J Lepr. 1956 Jan-Mar;24(1):65–73. [PubMed] [Google Scholar]
- Kaufmann S. H. Biological activities of a murine T-cell clone with reactivity to Mycobacterium leprae. Cell Immunol. 1984 Jan;83(1):215–220. doi: 10.1016/0008-8749(84)90241-7. [DOI] [PubMed] [Google Scholar]
- Kohsaka K., Mori T., Ito T. Lepromatoid lesion developed in nude mouse inoculated with Mycobacterium leprae--animal transmission of leprosy. Repura. 1976 Jul-Sep;45(3):177–187. doi: 10.5025/hansen1930.45.3_177. [DOI] [PubMed] [Google Scholar]
- Lowe C., Brett S. J., Rees R. J. Adoptive cell transfer of resistance to Mycobacterium leprae infections in mice. Clin Exp Immunol. 1985 Aug;61(2):336–342. [PMC free article] [PubMed] [Google Scholar]
- Patel P. J., Lefford M. J. Specific and nonspecific resistance in mice immunized with irradiated Myobacterium leprae. Infect Immun. 1978 Jun;20(3):692–697. doi: 10.1128/iai.20.3.692-697.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prabhakaran K., Harris E. B., Kirchheimer W. F. Binding of 14C-labeled dopa by Mycobacterium leprae in vitro. Int J Lepr Other Mycobact Dis. 1976 Jan-Jun;44(1-2):58–64. [PubMed] [Google Scholar]
- Shannon E. J., Miranda R. O., Morales M. J., Hastings R. C. Inhibition of de novo IgM antibody synthesis by thalidomide as a relevant mechanism of action in leprosy. Scand J Immunol. 1981;13(6):553–562. doi: 10.1111/j.1365-3083.1981.tb00169.x. [DOI] [PubMed] [Google Scholar]
- Shepard C. C., McRae D. H. A method for counting acid-fast bacteria. Int J Lepr Other Mycobact Dis. 1968 Jan-Mar;36(1):78–82. [PubMed] [Google Scholar]
- Shepard C. C., Walker L. L., Van Landingham R. M. Immunity to Mycobacterium leprae infections induced in mice by BCG vaccination at different times before or after challenge. Infect Immun. 1978 Feb;19(2):391–394. doi: 10.1128/iai.19.2.391-394.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]