Abstract
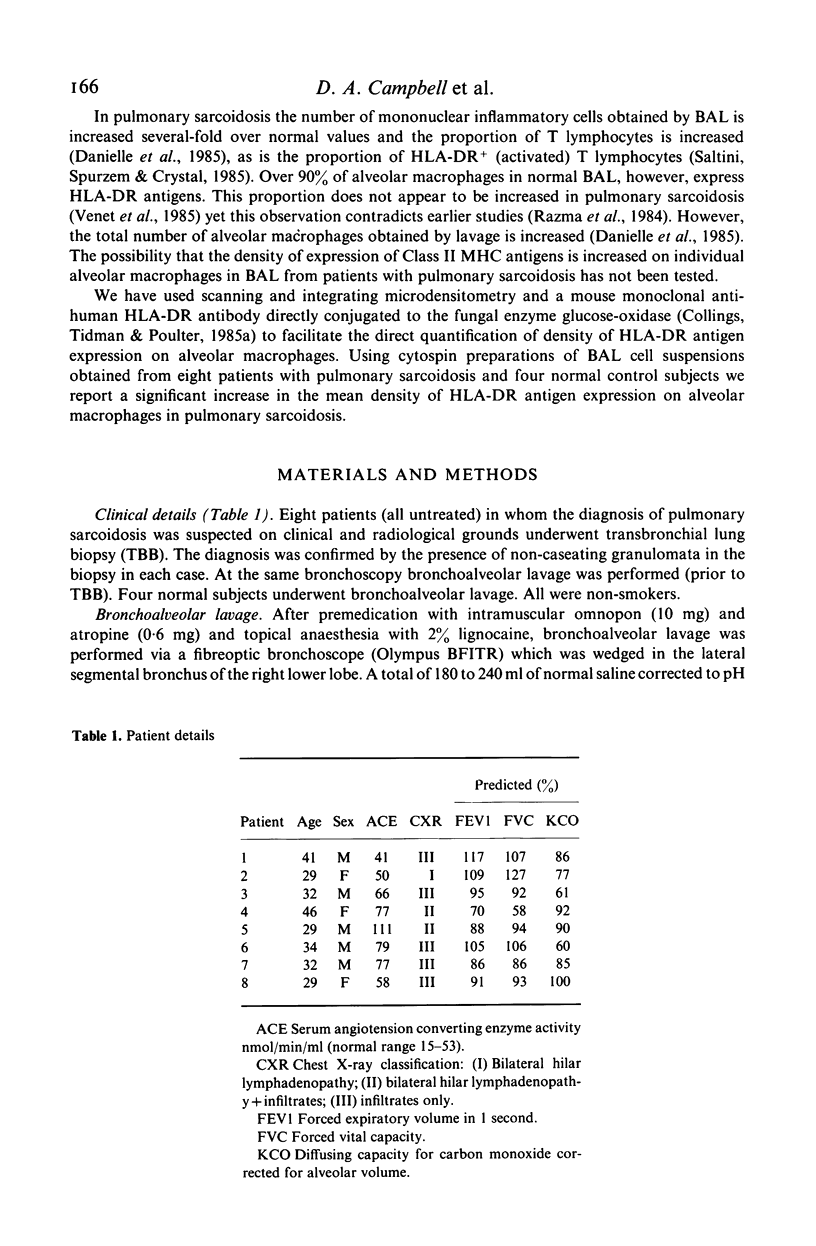
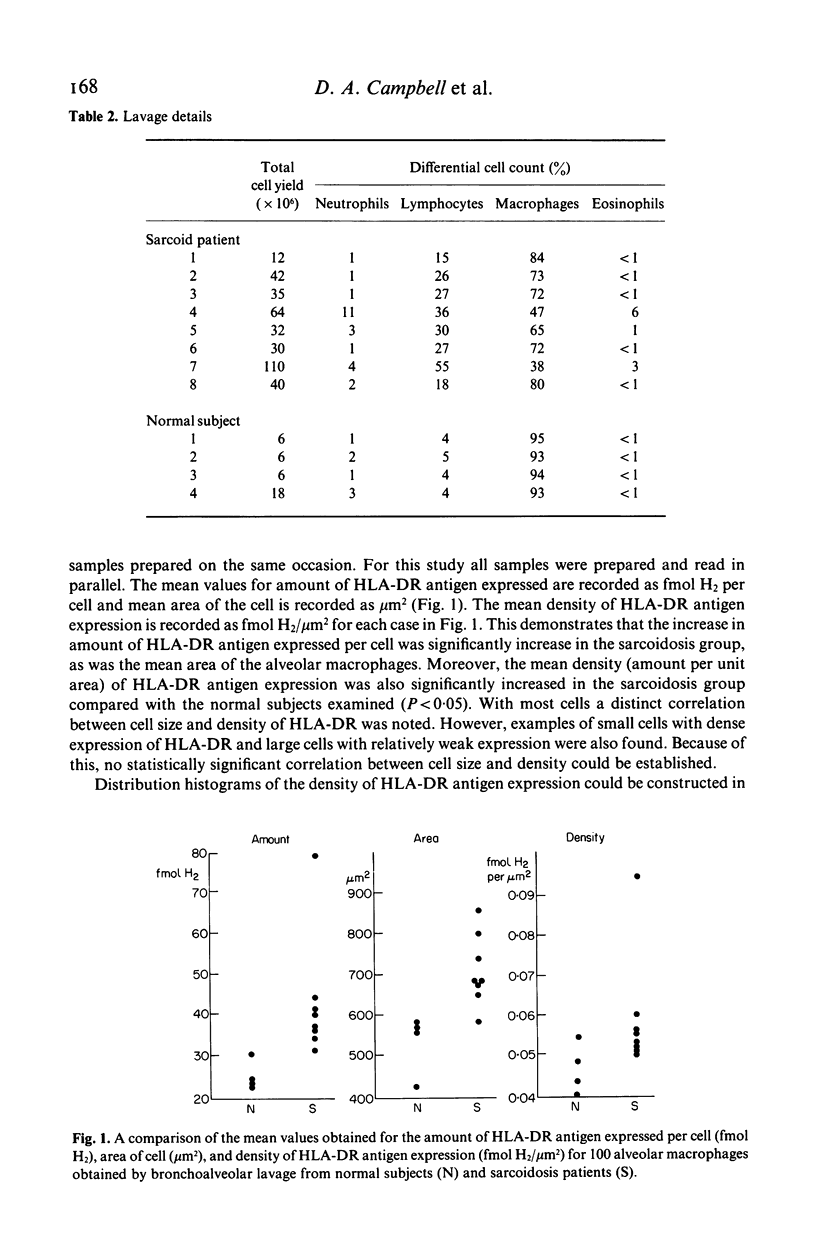
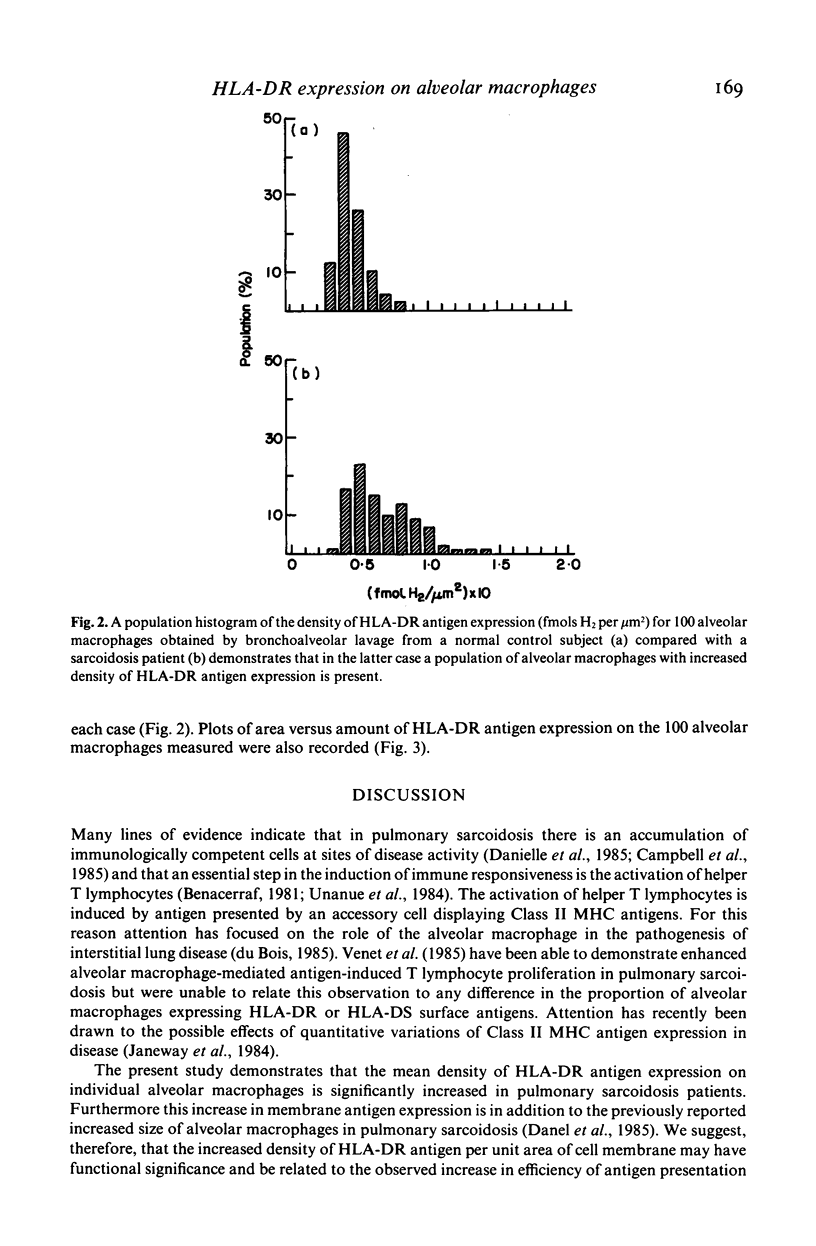
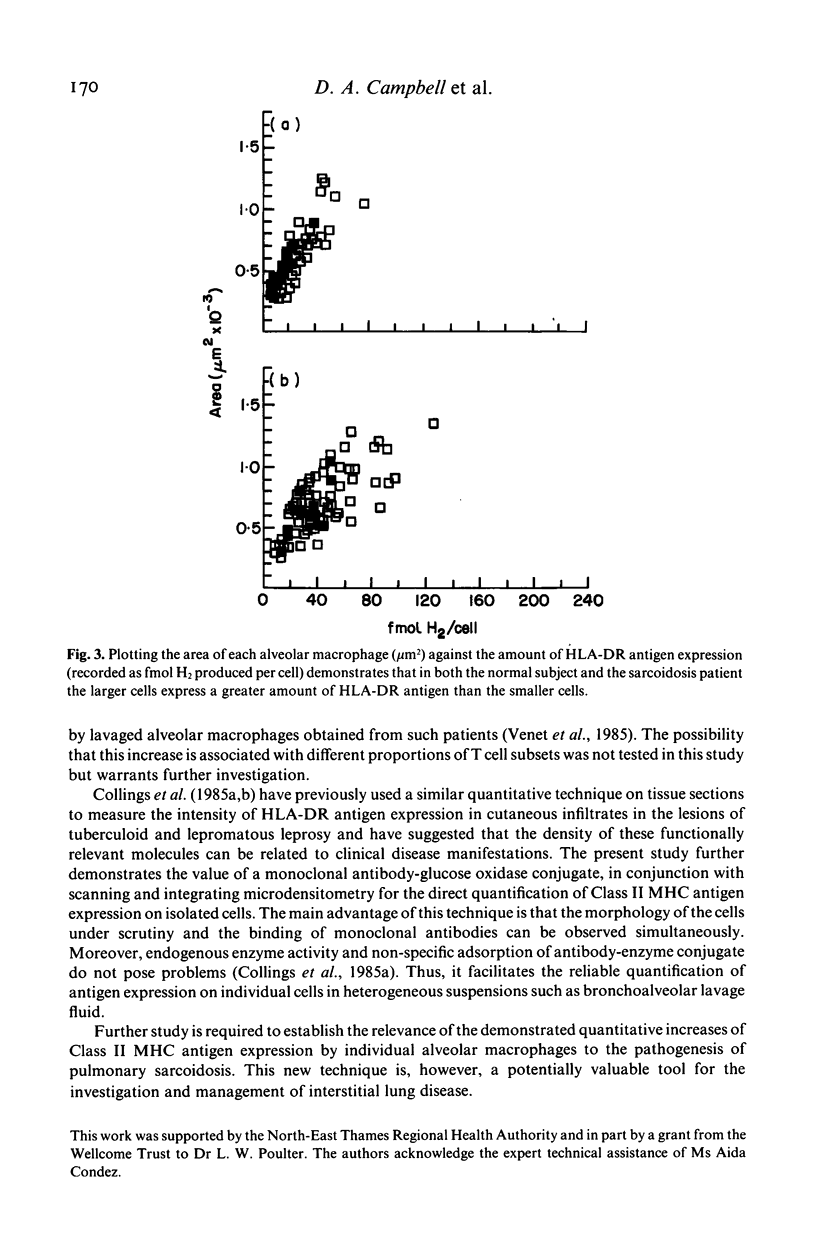
The density of HLA-DR antigen expression on alveolar macrophages obtained by bronchoalveolar lavage from sarcoidosis patients and normal control subjects was quantified using scanning and integrating microdensitometry in conjunction with a monoclonal mouse anti-human HLA-DR antibody directly conjugated to fungal glucose oxidase. The density of HLA-DR antigen expression on alveolar macrophages was significantly increased in the pulmonary sarcoidosis patients compared with normal control subjects examined. The reproducibility, specificity and sensitivity establish the reliability and potential importance of this method of direct quantification of cell surface antigen expression. The demonstration of an increased density of Class II Major Histocompatibility Complex (MHC) antigen expression on alveolar macrophages in pulmonary sarcoidosis suggests that these cells may play a role in the induction of the immune responses at sites of disease activity in this poorly understood condition.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benacerraf B. Role of MHC gene products in immune regulation. Science. 1981 Jun 12;212(4500):1229–1238. doi: 10.1126/science.6165083. [DOI] [PubMed] [Google Scholar]
- Butcher R. G., Altman F. P. Studies on the reduction of tetrazolium salts. II. The measurement of the half reduced and fully reduced formazans of neotetrazolium chloride in tissue sections. Histochemie. 1973 Dec 31;37(4):351–363. doi: 10.1007/BF00274970. [DOI] [PubMed] [Google Scholar]
- Butcher R. G. Precise cytochemical measurement of neotetrazolium formazan by scanning and integrating microdensitometry. Histochemie. 1972;32(2):171–190. doi: 10.1007/BF00303732. [DOI] [PubMed] [Google Scholar]
- Butcher R. G. The measurement in tissue sections of the two formazans derived from nitroblue tetrazolium in dehydrogenase reactions. Histochem J. 1978 Nov;10(6):739–744. doi: 10.1007/BF01003123. [DOI] [PubMed] [Google Scholar]
- Campbell D. A., Poulter L. W., du Bois R. M. Immunocompetent cells in bronchoalveolar lavage reflect the cell populations in transbronchial biopsies in pulmonary sarcoidosis. Am Rev Respir Dis. 1985 Dec;132(6):1300–1306. doi: 10.1164/arrd.1985.132.6.1300. [DOI] [PubMed] [Google Scholar]
- Collings L. A., Tidman N., Poulter L. W. Quantitation of HLA-DR expression by cells involved in the skin lesions of tuberculoid and lepromatous leprosy. Clin Exp Immunol. 1985 Jul;61(1):58–66. [PMC free article] [PubMed] [Google Scholar]
- Danel C., Dewar A., Corrin B., Turner-Warwick M., Chretien J. Ultrastructural changes in bronchoalveolar lavage cells in sarcoidosis and comparison with the tissue granuloma. Am J Pathol. 1983 Jul;112(1):7–17. [PMC free article] [PubMed] [Google Scholar]
- Daniele R. P., Elias J. A., Epstein P. E., Rossman M. D. Bronchoalveolar lavage: role in the pathogenesis, diagnosis, and management of interstitial lung disease. Ann Intern Med. 1985 Jan;102(1):93–108. doi: 10.7326/0003-4819-102-1-93. [DOI] [PubMed] [Google Scholar]
- Haslam P. L., Turton C. W., Heard B., Lukoszek A., Collins J. V., Salsbury A. J., Turner-Warwick M. Bronchoalveolar lavage in pulmonary fibrosis: comparison of cells obtained with lung biopsy and clinical features. Thorax. 1980 Jan;35(1):9–18. doi: 10.1136/thx.35.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunninghake G. W. Release of interleukin-1 by alveolar macrophages of patients with active pulmonary sarcoidosis. Am Rev Respir Dis. 1984 Apr;129(4):569–572. [PubMed] [Google Scholar]
- Poulter L. W. Antigen presenting cells in situ: their identification and involvement in immunopathology. Clin Exp Immunol. 1983 Sep;53(3):513–520. [PMC free article] [PubMed] [Google Scholar]
- Razma A. G., Lynch J. P., 3rd, Wilson B. S., Ward P. A., Kunkel S. L. Expression of Ia-like (DR) antigen on human alveolar macrophages isolated by bronchoalveolar lavage. Am Rev Respir Dis. 1984 Mar;129(3):419–424. doi: 10.1164/arrd.1984.129.3.419. [DOI] [PubMed] [Google Scholar]
- Steeg P. S., Sztein M. B., Mann D. L., Strong D. M., Oppenheim J. J. Interferon regulation of DR antigen expression and alloantigen-presenting capabilities of the promyelocytic cell line HL60. Scand J Immunol. 1985 May;21(5):425–430. doi: 10.1111/j.1365-3083.1985.tb01828.x. [DOI] [PubMed] [Google Scholar]
- Unanue E. R., Beller D. I., Lu C. Y., Allen P. M. Antigen presentation: comments on its regulation and mechanism. J Immunol. 1984 Jan;132(1):1–5. [PubMed] [Google Scholar]
- Venet A., Hance A. J., Saltini C., Robinson B. W., Crystal R. G. Enhanced alveolar macrophage-mediated antigen-induced T-lymphocyte proliferation in sarcoidosis. J Clin Invest. 1985 Jan;75(1):293–301. doi: 10.1172/JCI111688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- du Bois R. M. The alveolar macrophage. Thorax. 1985 May;40(5):321–327. doi: 10.1136/thx.40.5.321. [DOI] [PMC free article] [PubMed] [Google Scholar]



