Abstract
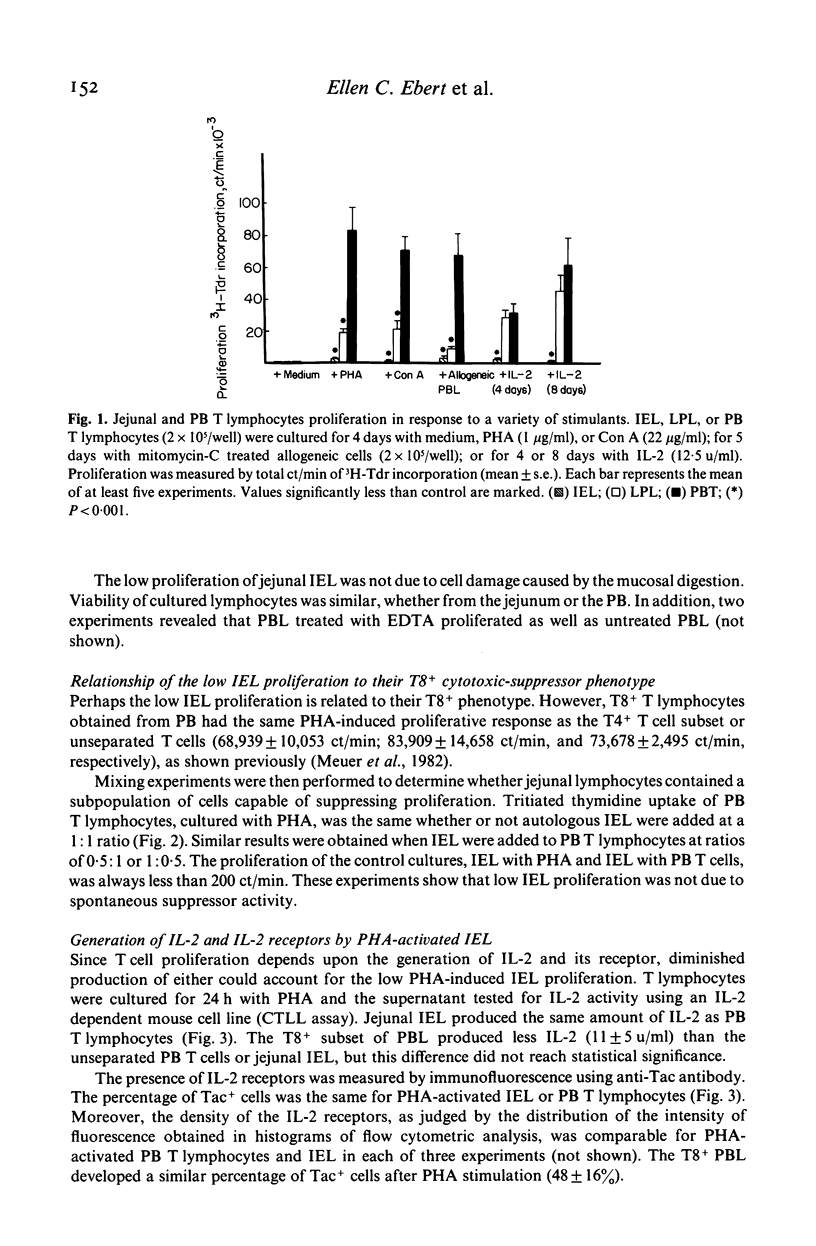
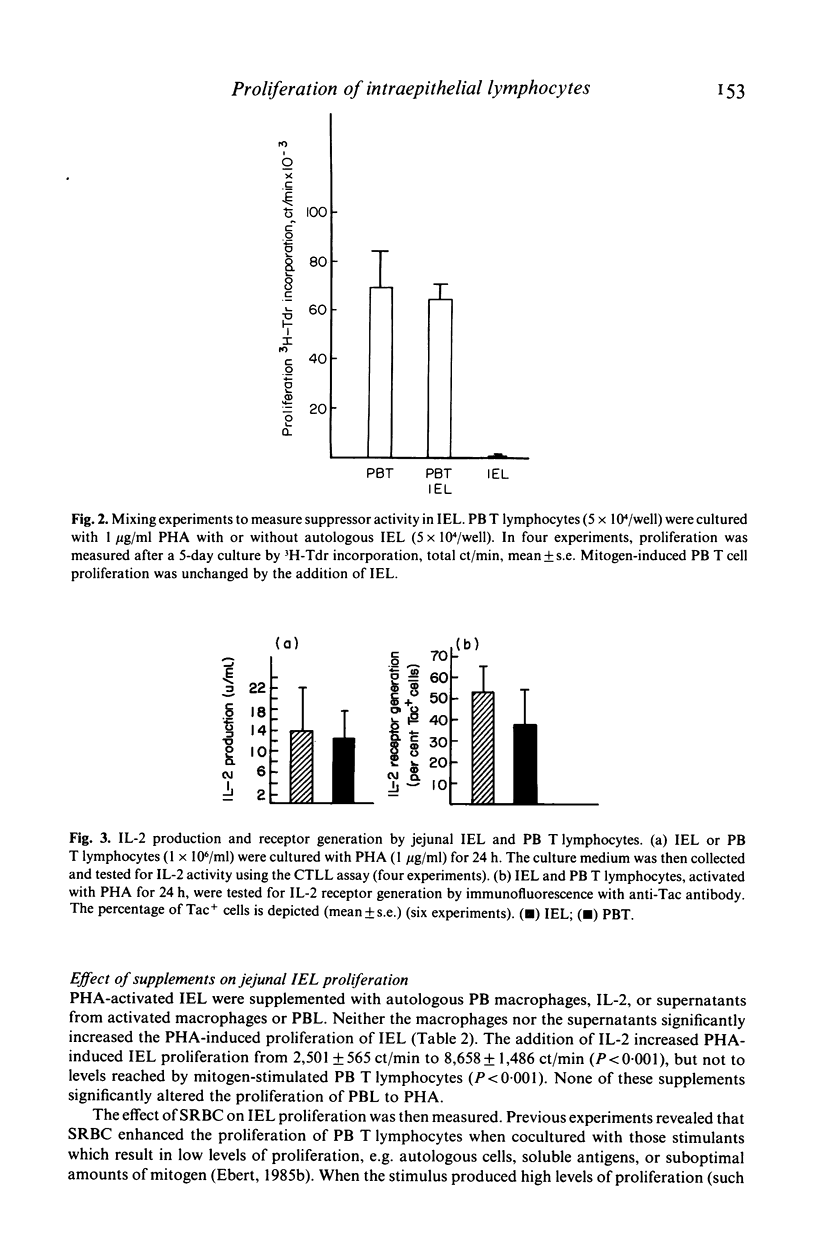
The proliferation of human jejunal intraepithelial lymphocytes (IEL) was examined to determine how it differed from that of peripheral blood (PB) T lymphocytes. The IEL were mainly T lymphocytes of the cytotoxic-suppressor (T8+) phenotype. They demonstrated lower proliferative responses to various stimuli (2,501 +/- 565 ct/min with phytohaemagglutinin; PHA) compared to unseparated PB T lymphocytes (73,678 +/- 2,495) or the T8+ subset (68,939 +/- 10,053 ct/min) (P less than 0.001). This low proliferative response was also a characteristic of the T8+ T lymphocytes in the lamina propria (4,606 +/- 1,226 ct/min) but not the T4+ subset (43,447 +/- 10,188 ct/min) (P less than 0.05). These findings were not due to isolation techniques or to differences in kinetics. Mixing experiments revealed that the IEL did not contain cells which suppressed proliferation. In addition, the IEL could be stimulated by mitogens, as they produced the same amount of interleukin 2 (IL-2) and IL-2 receptors as did PB T lymphocytes. Although the lectin-induced proliferative response of IEL was unaltered by the addition of autologous macrophages and minimally increased by IL-2, it was markedly enhanced by the addition of sheep red blood cells (SRBC). The enhancing effect of SRBC was not due to T cell recognition of xenogenic antigens on the erythrocytes since neither allogeneic non-T lymphocytes nor other xenogenic erythrocytes produced the same effect. Both intact SRBC and membrane fragments from osmotically lysed cells augmented lymphocyte proliferation. Thus, jejunal IEL could be activated by mitogen and proliferated as much as PB T lymphocytes if exposed to a membrane component found on SRBC.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bland P. W., Britton D. C., Richens E. R., Pledger J. V. Peripheral, mucosal, and tumour-infiltrating components of cellular immunity in cancer of the large bowel. Gut. 1981 Sep;22(9):744–751. doi: 10.1136/gut.22.9.744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bookman M. A., Bull D. M. Characteristics of isolated intestinal mucosal lymphoid cells in inflammatory bowel disease. Gastroenterology. 1979 Sep;77(3):503–510. [PubMed] [Google Scholar]
- Bull D. M., Bookman M. A. Isolation and functional characterization of human intestinal mucosal lymphoid cells. J Clin Invest. 1977 May;59(5):966–974. doi: 10.1172/JCI108719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerf-Bensussan N., Guy-Grand D., Griscelli C. Intraepithelial lymphocytes of human gut: isolation, characterisation and study of natural killer activity. Gut. 1985 Jan;26(1):81–88. doi: 10.1136/gut.26.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerf-Bensussan N., Schneeberger E. E., Bhan A. K. Immunohistologic and immunoelectron microscopic characterization of the mucosal lymphocytes of human small intestine by the use of monoclonal antibodies. J Immunol. 1983 Jun;130(6):2615–2622. [PubMed] [Google Scholar]
- Eade O. E., Andre-Ukena S. S., Moulton C., MacPherson B., Beeken W. L. Lymphocyte subpopulations of intestinal mucosa in inflammatory bowel disease. Gut. 1980 Aug;21(8):675–682. doi: 10.1136/gut.21.8.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebert E. C. Sheep red blood cells enhance T-lymphocyte proliferation. Clin Immunol Immunopathol. 1985 Nov;37(2):203–212. doi: 10.1016/0090-1229(85)90151-5. [DOI] [PubMed] [Google Scholar]
- Ebert E. C., Stoll D. B., Cassens B. J., Lipshutz W. H., Hauptman S. P. Diminished interleukin 2 production and receptor generation characterize the acquired immunodeficiency syndrome. Clin Immunol Immunopathol. 1985 Dec;37(3):283–297. doi: 10.1016/0090-1229(85)90099-6. [DOI] [PubMed] [Google Scholar]
- Ebert E. C., Wright S. H., Lipshutz W. H., Hauptman S. P. T-cell abnormalities in inflammatory bowel disease are mediated by interleukin 2. Clin Immunol Immunopathol. 1984 Nov;33(2):232–244. doi: 10.1016/0090-1229(84)90078-3. [DOI] [PubMed] [Google Scholar]
- Ferguson A. Intraepithelial lymphocytes of the small intestine. Gut. 1977 Nov;18(11):921–937. doi: 10.1136/gut.18.11.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson A., McClure J. P., Townley R. R. Intraepithelial lymphocyte counts in small intestinal biopsies from children with diarrhoea. Acta Paediatr Scand. 1976 Sep;65(5):541–546. doi: 10.1111/j.1651-2227.1976.tb04929.x. [DOI] [PubMed] [Google Scholar]
- Fiocchi C., Hilfiker M. L., Youngman K. R., Doerder N. C., Finke J. H. Interleukin 2 activity of human intestinal mucosa mononuclear cells. Decreased levels in inflammatory bowel disease. Gastroenterology. 1984 Apr;86(4):734–742. [PubMed] [Google Scholar]
- Gillis S., Ferm M. M., Ou W., Smith K. A. T cell growth factor: parameters of production and a quantitative microassay for activity. J Immunol. 1978 Jun;120(6):2027–2032. [PubMed] [Google Scholar]
- Goodacre R. L., Bienenstock J. Reduced suppressor cell activity in intestinal lymphocytes from patients with Crohn's disease. Gastroenterology. 1982 Apr;82(4):653–658. [PubMed] [Google Scholar]
- Greenwood J. H., Austin L. L., Dobbins W. O., 3rd In vitro characterization of human intestinal intraepithelial lymphocytes. Gastroenterology. 1983 Nov;85(5):1023–1035. [PubMed] [Google Scholar]
- Julius M. H., Simpson E., Herzenberg L. A. A rapid method for the isolation of functional thymus-derived murine lymphocytes. Eur J Immunol. 1973 Oct;3(10):645–649. doi: 10.1002/eji.1830031011. [DOI] [PubMed] [Google Scholar]
- Lopez-Botet M., Moretta L. Functional characterization of human thymocytes: a limiting dilution analysis of precursors with proliferative and cytolytic activities. J Immunol. 1985 Apr;134(4):2299–2304. [PubMed] [Google Scholar]
- MacDermott R. P., Bragdon M. J., Jenkins K. M., Franklin G. O., Shedlofsky S., Kodner I. J. Human intestinal mononuclear cells. II. Demonstration of a naturally occurring subclass of T cells which respond in the allogeneic mixed leukocyte reaction but do not effect cell-mediated lympholysis. Gastroenterology. 1981 Apr;80(4):748–757. [PubMed] [Google Scholar]
- Meuer S. C., Hussey R. E., Fabbi M., Fox D., Acuto O., Fitzgerald K. A., Hodgdon J. C., Protentis J. P., Schlossman S. F., Reinherz E. L. An alternative pathway of T-cell activation: a functional role for the 50 kd T11 sheep erythrocyte receptor protein. Cell. 1984 Apr;36(4):897–906. doi: 10.1016/0092-8674(84)90039-4. [DOI] [PubMed] [Google Scholar]
- Meuer S. C., Hussey R. E., Penta A. C., Fitzgerald K. A., Stadler B. M., Schlossman S. F., Reinherz E. L. Cellular origin of interleukin 2 (IL 2) in man: evidence for stimulus-restricted IL 2 production by T4+ and T8+ T lymphocytes. J Immunol. 1982 Sep;129(3):1076–1079. [PubMed] [Google Scholar]
- Montgomery R. D., Shearer A. C. The cell population of the upper jejunal mucosa in tropical sprue and postinfective malabsorption. Gut. 1974 May;15(5):387–391. doi: 10.1136/gut.15.5.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips A. D., Rice S. J., France N. E., Walker-Smith J. A. Small intestinal intraepithelial lymphocyte levels in cow's milk protein intolerance. Gut. 1979 Jun;20(6):509–512. doi: 10.1136/gut.20.6.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robb R. J., Greene W. C., Rusk C. M. Low and high affinity cellular receptors for interleukin 2. Implications for the level of Tac antigen. J Exp Med. 1984 Oct 1;160(4):1126–1146. doi: 10.1084/jem.160.4.1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selby W. S., Janossy G., Bofill M., Jewell D. P. Intestinal lymphocyte subpopulations in inflammatory bowel disease: an analysis by immunohistological and cell isolation techniques. Gut. 1984 Jan;25(1):32–40. doi: 10.1136/gut.25.1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selby W. S., Janossy G., Jewell D. P. Immunohistological characterisation of intraepithelial lymphocytes of the human gastrointestinal tract. Gut. 1981 Mar;22(3):169–176. doi: 10.1136/gut.22.3.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagliabue A., Luini W., Soldateschi D., Boraschi D. Natural killer activity of gut mucosal lymphoid cells in mice. Eur J Immunol. 1981 Nov;11(11):919–922. doi: 10.1002/eji.1830111112. [DOI] [PubMed] [Google Scholar]



