Abstract
Unmethylated CpG dinucleotides present within certain specific sequence contexts in bacterial and synthetic DNA stimulate innate immune responses and induce cytokine secretion. Recently, we showed that CpG DNAs containing two 5′-ends, immunomers, are more potent in both regards. In this study, we show that an immunomer containing a synthetic CpR motif (R = 2′-deoxy-7-deazaguanosine) is a potent immunostimulatory agent. However, the profile of cytokine induction is different from that with immunomers containing a natural CpG motif. In general, a CpR immunomer induced higher interleukin (IL)-12 and lower IL-6 secretion. Compared with conventional CpG DNAs, both types of immunomers showed a rapid and enhanced activation of the transcription factor NF-κB in J774 cells. NF-κB activation by CpG DNA corresponded to degradation of IκBα in J774 cells. All three immunostimulatory oligonucleotides activated the p38 mitogen-activated protein kinase pathway as expected. Immunomers containing CpG and CpR motifs showed potent reversal of the antigen-induced Th2 immune response towards a Th1 type in antigen-sensitized mouse spleen cell cultures. Immunomers containing a CpR motif showed significant antitumor activity in nude mice bearing MCF-7 human breast cancer and U87MG glioblastoma xenografts. These studies suggest the ability for a divergent synthetic nucleotide motif recognition pattern of the receptor involved in the immunostimulatory pathway and the possibility of using synthetic nucleotides to elicit different cytokine response patterns.
INTRODUCTION
The presence of CpG dinucleotides in certain sequence contexts in bacterial and synthetic oligodeoxyribonucleotides (CpG DNAs) is known to activate vertebrate innate immune cells and B cells (1–4). Various cytokines are secreted in the process, including interferon-γ (IFN-γ), interleukin (IL)-12, tumor necrosis factor-α (TNF-α) and IL-6, and the levels of co-stimulatory surface molecules increased (5–8). These activities depend on the sequences flanking the CpG dinucleotide (3,9–11), and we have investigated the structural and chemical characteristics of CpG DNA required for immune stimulation (12–14).
Although direct evidence for physical binding is lacking, it is believed that Toll-like receptor 9 (TLR9) recognizes CpG dinucleotides in bacterial and synthetic DNA and activates intracellular immune signaling pathways (15). Our structure– immunostimulatory activity studies showed that the receptor is highly specific for deoxyribonucleotides in CpG, as ribonucleotides or 2′-O-alkyl ribonucleotides abrogate immunostimulation (12). We have identified critical structural features in the pentose sugar (13,16–19), phosphate backbone (13,20), nucleobases (21,22) and nucleosides (23) required for activity. Moreover, through the use of synthetic pyrimidine (Y) and purine (R) bases in YpG and CpR motifs, we showed requirements for a functional group on cytosine and guanine in receptor recognition (24).
More importantly, our recent studies suggest that the receptor reads CpG DNA sequence from the 5′- to the 3′-end, and modifications that block the 5′-end abrogate immunostimulation (25–29). In contrast, CpG oligonucleotides with two accessible 5′-ends (immunomers) show enhanced activity (25–29). Immunomers containing phosphodiester backbones show enhanced nuclease resistance and immunostimulation without the need for palindromic or guanine-rich sequences (28). The size of ligand conjugated at the 5′-end determines accessibility. For example, fluorescein conjugation at the 5′-end does not effect cellular uptake compared with 3′-conjugate, but prevents immunostimulation (26). Immunomers containing as few as 5 or 6 nt in each segment also show potent immunostimulation in both mouse and human systems (29).
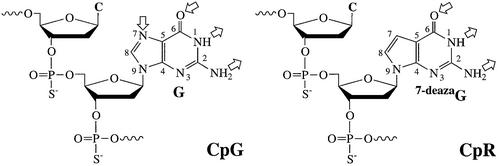
In the present study, we demonstrate that an immunomer shows increased immunostimulatory activity compared with a conventional CpG DNA molecule containing the same ‘GACGTT’ motif but only one 5′-terminus. Moreover, for the first time, we show that an immunomer containing a synthetic dinucleotide CpR (R = 2′-deoxy-7-deazaguanosine) (Fig. 1) exhibits potent immunostimulatory activity. Interest ingly, subtle differences were observed in cytokine secretion profiles induced by immunomers containing CpR and CpG motifs. These studies suggest that receptor response may be determined by nucleotide motif recognition patterns (NMRPs) and the receptor can recognize a variety of structurally similar nucleotide motifs.
Figure 1.
Structures of natural CpG and synthetic CpR immunostimulatory motifs. G and R are shown for comparison. Hydrogen bond donor and acceptor groups are shown with outward and inward arrows, respectively.
MATERIALS AND METHODS
Synthesis and purification
CpG DNA [1, 5′-d(CTATCTGACGTTCTCTGT)-3′] and immunomers [2, 5′-d(TCTGACGTTCT-X-TCTTGCAGTCT)-5′; 3, 5′-d(TCTGACRTTCT-X-TCTTRCAGTCT)-5′ where X and R are glyceryl linker and 2′-deoxy-7-deazaguanosine, respectively] were synthesized on a 1–2 µmol scale using β-cyanoethylphosphoramidite chemistry on a PerSeptive Biosystem’s 8909 Expedite DNA synthesizer as described earlier (27). The phosphoramidites of dA, dG, dC and T were obtained from Applied Biosystems. 2′-Deoxy-7-deazaguanosine phosphoramidite and the solid support attached with di-4,4′-dimethoxytrityl-protected glyceryl linker were obtained from ChemGenes. Beaucage reagent was used as an oxidant to obtain the phosphorothioate backbone modification (30). After the synthesis, immunomers were deprotected using standard protocols, purified by HPLC, and dialyzed against USP quality sterile water for irrigation (Braun). The immunomers were lyophilized and dissolved again in distilled water, and the concentrations were determined by measuring the UV absorbance at 260 nm (31). All the immunomers synthesized were characterized by capillary gel electrophoresis (CGE) and matrix-assisted laser desorption ionization time of flight mass spectrometry (Applied Biosystem’s Voyager-DE™ STR Biospectrometry™ Workstation) for purity and molecular mass, respectively. The purity of full-length immunomers ranged from 89 to 95%, with the rest being shorter by 1 or 2 nt (n – 1 and n – 2) as determined by CGE and/or denaturing PAGE. All CpG DNAs contained <0.1 EU/ml of endotoxin as determined by the Limulus assay (Bio-Whittaker).
Cell culture conditions and reagents
Spleen cells from 4- to 8-week-old BALB/c, C57BL/6 or C3H/HeJ mice were cultured in RPMI complete medium as described earlier (12,32). Murine J774 macrophage cells (American Type Culture Collection, Rockville, MD) were cultured in Dulbecco’s modified Eagle’s medium supplemented with 10% (v/v) fetal calf serum and antibiotics (100 IU/ml of penicillin G/streptomycin). All other culture reagents were purchased from Mediatech (Gaithersburg, MD).
Cytokine ELISAs
Mouse spleen or J774 cells were plated in 24-well dishes using 5 × 106 or 1 × 106 cells/ml, respectively. The CpG DNA dissolved in TE buffer (10 mM Tris–HCl pH 7.5, 1 mM EDTA) was added to a final concentration of 0.03, 0.1, 0.3, 1.0, 3.0 or 10.0 µg/ml to the cell cultures. The cells were then incubated at 37°C for 24 h and the supernatants were collected for enzyme-linked immunosorbent assays (ELISAs). The experiments were performed two or three times for each CpG DNA in triplicate for each concentration. The secretion of IL-12 and IL-6 was measured by sandwich ELISA as described previously (27). The required reagents, including cytokine antibodies and standards, were purchased from PharMingen.
Mouse splenomegaly assay
Female BALB/c mice (4–6 weeks, 19–21 g) were divided into groups of two or three mice. CpG DNAs were dissolved in sterile phosphate-buffered saline (PBS) and administered intraperitoneally (i.p.) to mice at a dose of 10 mg/kg. The mice were sacrificed after 40 h, the spleens were harvested and weighed as described previously (12,32) and the number of mononuclear cells was determined. Blood was withdrawn from animals by retro-orbital puncture before sacrificing for cytokine assays. The IL-12 levels in serum samples were determined by ELISA as described above.
Sensitization of mice with conalbumin
Four- to six-week-old BALB/c female mice were obtained from Taconic (Germantown, NY). The mice were given i.p. injections of 200 µg of conalbumin (Sigma) in 100 µl of PBS mixed with 100 µl of ImjectAlum adjuvant (Pierce) on days 0 and 7, and intranasally challenged on days 14 and 21. The mice were sacrificed 72 h after the last challenge by CO2 inhalation. Spleens were excised and single cell suspensions were prepared as described above. Spleen cells were treated with immunomers at different concentrations for 2 h followed by treatment with 50 µg/ml of conalbumin. Supernatants were harvested after 72 h, and IL-5, IL-6 and IL-12 levels were measured by ELISA as described above.
Preparation of J774 cell nuclear extracts and EMSA
NF-κB activation in J774 cells treated with CpG DNAs was carried out and analyzed by EMSA as described previously (27).
Preparation of J774 cell lysates and western blotting
The levels of phosphorylated and total p38 mitogen-activated protein kinases (MAPKs) in J774 cells following CpG DNA treatment were measured by western blotting as described previously (28). All antibodies required for the assay were purchased from Cell Signaling Technology.
In vivo nude mice human cancer models and treatment plan
The animal use and care protocols were approved by the Institutional Committee on Animal Use and Care of the University of Alabama at Birmingham. Female athymic nude mice (nu/nu, 4–6 weeks old) were obtained from the Frederick Cancer Research and Development Center (Frederick, MD, USA) and inoculated with MCF-7 or U87MG cells as described previously (33,34). Tumor-bearing animals were divided randomly into various treatment groups (five/group) and treated by subcutaneous injection with immunomer 3 at a dose of 0.5 mg/kg/injection or saline (control) 3 days per week (days 1, 3 and 5). The MCF-7 study was terminated on day 15 and the U87MG study was terminated on day 42 when the tumor size reached 3000 mg in control group mice. Animals in the treatment group in the MCF-7 study received a total of six injections and those in the U87MG study received a total of 15 injections. The mice were monitored by general clinical observations, body weight and tumor growth. Tumor growth was recorded with the use of calipers, by measuring the long and short diameters of the tumor. Tumor mass (in g) was calculated using the formula 1/2a × b2, where a and b are the long and short diameters (in cm), respectively.
RESULTS
CpG DNA containing two accessible 5′-ends shows greater immune stimulation
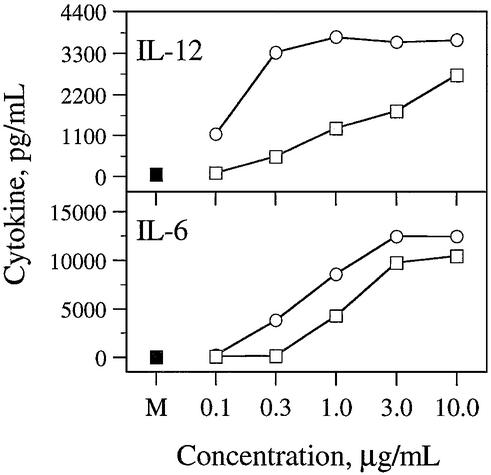
Activation of mouse (BALB/c, C57BL/6 or C3H/HeJ) spleen cells in cultures with CpG DNA for 24 h resulted in cytokine induction. In the present studies, two typical cytokines, IL-12 and IL-6, are measured. Both 1 and 2 induced concentration-dependent IL-12 and IL-6 secretion in all three strains of mouse spleen cell cultures (Table 1). Figure 2 shows a typical concentration-dependent IL-12 and IL-6 secretion in BALB/c mouse spleen cell cultures. In general, 3′–3′-linked immunomer 2 induced higher IL-12 secretion than conventional CpG DNA 1. A control DNA molecule lacking CpG induced similar levels of IL-12 and IL-6 secretion to background values observed with medium alone (data not shown). Previously we showed that two CpG DNAs covalently attached through 5′–5′ linkages induce minimal cytokine secretion (25–28). We also demonstrated that two CpG DNAs attached through 5′–3′ linkage (containing two immunostimulatory motifs) showed no greater activity than a shorter CpG DNA containing one immunostimulatory motif (27). An undecanucleotide that corresponds to one of the segments of immunomer 2 showed minimal activity in previous studies (27). Therefore, 5′–5′-linked and 5′–3′-linked oligonucleotides were not tested further in the present study.
Table 1. Induction of cytokine secretion by immunomers in mouse spleen cell cultures.
| CpG DNA | IL-12 (pg/ml) ± SD | IIL-6 (pg/ml) ± SD | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.1 µg/ml | 0.3 µg/ml | 1.0 µg/ml | 3.0 µg/ml | 10.0 µg/ml | 0.1 µg/ml | 0.3 µg/ml | 1.0 µg/ml | 3.0 µg/ml | 10.0 µg/ml | |||||||||||
| BALB/c | ||||||||||||||||||||
| 1 | 100 ± 3 | 546 ± 23 | 1313 ± 69 | 1775 ± 139 | 2732 ± 193 | 90 ± 0 | 162 ± 16 | 4316 ± 330 | 9774 ± 798 | 10 472 ± 264 | ||||||||||
| 2 | 1142 ± 99 | 3330 ± 324 | 3735 ± 180 | 3604 ± 186 | 3656 ± 76 | 240 ± 57 | 3836 ± 169 | 8601 ± 266 | 12 507 ± 177 | 12 468 ± 252 | ||||||||||
| 3 | 219 ± 9 | 1151 ± 30 | 2601 ± 197 | 2823 ± 134 | 4351 ± 113 | 11 ± 3 | 462 ± 32 | 4230 ± 31 | 10 498±438 | 11 890 ± 291 | ||||||||||
| C | 57 ± 4 | 7 ± 1.6 | ||||||||||||||||||
| C57BL/6 | ||||||||||||||||||||
| 1 | 130 ± 8 | 682 ± 2 | 787 ± 19 | 889 ± 71 | 948 ± 109 | 332 ± 88 | 1006 ± 125 | 6398 ± 536 | 7257 ± 132 | 9860 ± 964 | ||||||||||
| 2 | 1526 ± 171 | 1989 ± 272 | 1771 ± 41 | 1608 ± 38 | 1330 ± 158 | 1069 ± 59 | 5944 ± 167 | 12 390 ± 932 | 9849 ± 734 | 12 224 ± 131 | ||||||||||
| 3 | 454 ± 23 | 1528 ± 56 | 1478 ± 90 | 1487 ± 46 | 1816 ± 107 | 54 ± 12 | 1848 ± 154 | 8770 ± 659 | 11 149 ± 1556 | 13 701 ± 1660 | ||||||||||
| C | 71 ± 11 | 108 ± 38 | ||||||||||||||||||
| C3H/HeJ | ||||||||||||||||||||
| 1 | 206 ± 23 | 781 ± 63 | 890 ± 53 | 931 ± 148 | 1120 ± 43 | ND | 466 ± 42 | 2546 ± 204 | 5465 ± 140 | 6134 ± 171 | ||||||||||
| 2 | 1394 ± 97 | 2183 ± 96 | 1935 ± 115 | 1770 ± 33 | 1433 ± 74 | 222 ± 23 | 1556 ± 35 | 7910 ± 182 | 8887 ± 171 | 7669 ± 251 | ||||||||||
| 3 | 487 ± 35 | 1124 ± 160 | 1428 ± 55 | 1414 ± 71 | 1484 ± 101 | 20 ± 0 | 405 ± 64 | 2712 ± 92 | 6686 ± 189 | 6953 ± 201 | ||||||||||
| C | 25 ± 6 | 10 ± 3 | ||||||||||||||||||
C, no added immunomer control; ND, not detected.
Figure 2.
Induction of cytokine secretion by CpG DNAs in BALB/c mouse spleen cell cultures. BALB/c mouse spleen cells were cultured and activated with CpG DNAs 1 (squares) and 2 (circles) at various concentrations for 24 h, and the levels of secreted IL-12 (top) and IL-6 (bottom) in the culture medium were measured by ELISA. See Materials and Methods for cell culture and ELISA protocols. M stands for medium-treated control (filled square). Each value is an average of three replicates, and the results are representative of at least two independent experiments.
A synthetic CpR motif shows potent immunostimulatory activity
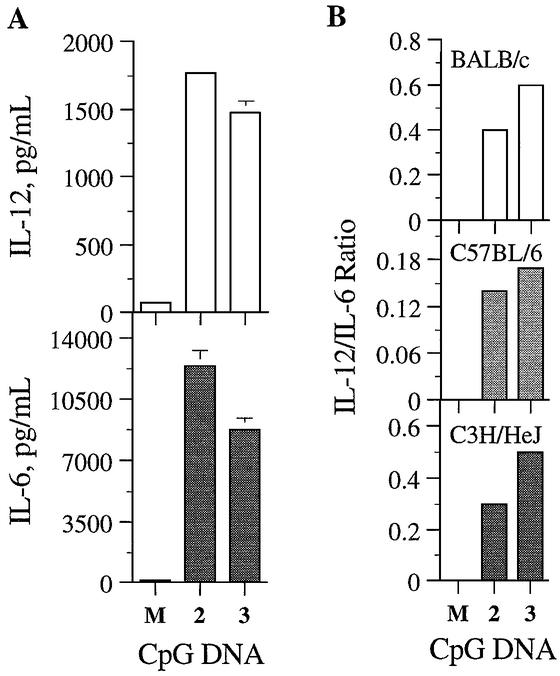
We substituted the G of the CpG dinucleotide in 2 with R (2′-deoxy-7-deazaguanosine) to obtain compound 3 containing a synthetic immunostimulatory motif CpR (Fig. 1). In all three strains of mouse spleen cell culture assays, 3 induced IL-12 and IL-6 secretion (Table 1), suggesting that the presence of R in immunomers does not prevent binding to the receptor. Figure 3A shows the results obtained with 2 and 3 in C57BL/6 mouse spleen cell cultures. Surprisingly, activation of spleen cells with 3 resulted in similar levels of IL-12, but lower IL-6, induction compared with that with 2 in all three mouse strains tested (Table 1). The plots of ratios of IL-12 to IL-6 show the differences in cytokine induction profiles by 2 and 3 in all three strains of mouse spleen cells (Fig. 3B). Additionally, similar activity observed in lipopolysaccharide non-responsive C3H/HeJ mouse spleen cell cultures suggest that immunomers do not activate immune cells through TLR4.
Figure 3.
(A) Induction of cytokine secretion by immunomers in C57BL/6 mouse spleen cell cultures. C57BL/6 mouse spleen cells were cultured and activated with immunomers 2 and 3 at various concentrations for 24 h, and the levels of secreted IL-12 (top) and IL-6 (bottom) in the culture medium were measured by ELISA. The results shown are for 1 µg/ml concentration. See Materials and Methods for cell culture and ELISA protocols. M stands for medium-treated control. Each value is an average of three replicates, and the results are representative of at least two independent experiments. (B) The ratio of IL-12 to IL-6 induced at 1 µg/ml concentration of immunomers 2 and 3 in BALB/c, C57BL/6 and C3H/HeJ mouse spleen cell cultures.
In vivo effects of CpG DNAs on splenomegaly and serum IL-12 levels
To evaluate the immunostimulatory effects in vivo, CpG DNAs 1–3 were administered to BALB/c mice i.p. at a dose of 10 mg/kg (single dose). The increases in spleen weight, splenocyte number and IL-12 levels in serum were determined 40 h post administration (Table 2). A significant increase in spleen weights was observed in mice injected with CpG DNA compared with mice injected with PBS (86 ± 10 mg) (Fig. 4). The mice treated with CpG DNAs 1–3 showed an average increase of 300–330% in their spleen weights compared with the control group. There were also more mononuclear cells in spleens of treated mice (Table 2). After 40 h, serum IL-12 levels increased with all three CpG DNAs (Table 2). Moreover, as in cell cultures, greater IL-12 induction resulted with immunomers 2 and 3 than with 1.
Table 2. In vivo effects of conventional CpG DNA and immunomers in BALB/c micea.
| CpG DNA | Spleen weight (mg ± SD) | Mononuclear cells/spleen, ×106 (% expansion) | Serum IL-12 (pg/ml ± SD) |
|---|---|---|---|
| 1 | 259 ± 23 | 78 (13.0) | 2202 ± 220 |
| 2 | 274 ± 27 | 121 (75.4) | 4739 ± 293 |
| 3 | 284 ± 12 | 86 (24.6) | 5447 ± 253 |
| PBS | 86 ± 10 | 69 | ND |
aA single dose of 10 mg/kg was given i.p. and the data were obtained 40 h post-administration.
ND, not detected.
Figure 4.
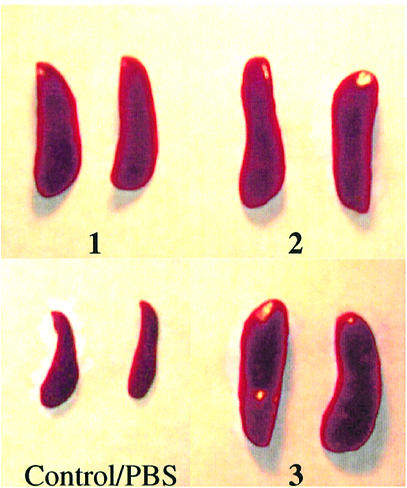
Administration of CpG DNA to mice causes splenomegaly. Photographs of representative spleens of BALB/c mice after 40 h of treatment with a single dose of CpG DNA 1–3 or PBS as described in Materials and Methods.
Activation of NF-κB in J774 cells
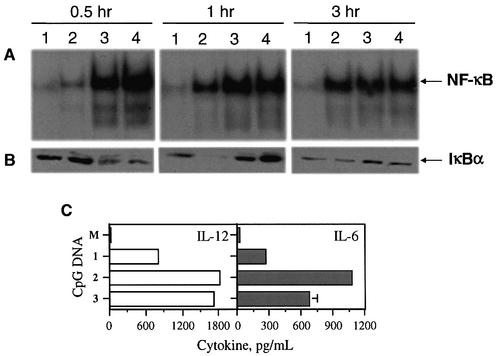
The transcription factor NF-κB is activated by CpG DNA treatment of macrophages (35) and is known to induce cytokine secretion. We compared the activation of NF-κB by CpG DNAs 1–3 in J774 cells. Figure 5A shows an EMSA of J774 nuclear extracts treated with an NF-κB-binding oligonucleotide probe. All three CpG DNAs activated the transcription factor, as shown in Figure 5B. However, significant differences are seen in the kinetics of activation of NF-κB (Fig. 5A) by 1 (lane 2) and immunomers 2 (lane 3) and 3 (lane 4). Immunomers 2 and 3 showed strong activation of NF-κB within 30 min, while 1 showed minimal activation in this time. After 1 h, significant activation of NF-κB was found with all three CpG DNAs 1–3. However, immunomers 2 and 3 showed relatively more potent activation of NF-κB at this time also compared with 1 (Fig. 5A). After 3 h, all three CpG DNAs showed a similarly strong NF-κB presence in J774 cell nuclear extracts. Importantly, both 2 and 3, containing natural CpG and synthetic CpR motifs, respectively, showed similar levels of NF-κB activation.
Figure 5.
(A) Activation of the transcription factor NF-κB in J774 macrophage cells upon treatment with CpG DNAs. J774 cell cultures were treated with PBS (lane 1) or 10 µg/ml of CpG DNAs 1 (lane 2), 2 (lane 3) or 3 (lane 4) for 0.5, 1 or 3 h, and nuclear extracts were isolated, treated with a 32P-labeled DNA probe containing NF-κB-binding sequence and analyzed by EMSA as described in Materials and Methods. (B) Degradation of IκBα in J774 macrophage cells upon treatment with CpG DNAs. J774 cell cultures were treated with PBS (lane 1) or 10 µg/ml of CpG DNAs 1 (lane 2), 2 (lane 3) or 3 (lane 4) for 0.5, 1 or 3 h, and cytoplasmic fractions were extracted, and analyzed as described in Materials and Methods. (C) Induction of cytokine secretion by immunomers in J774 macrophage cell cultures. J774 cells were cultured and activated with CpG DNAs 1–3 at various concentrations for 24 h and the levels of secreted IL-12 (left) and IL-6 (right) in the culture medium were measured by ELISA. The results shown are for 10 µg/ml concentration. See Materials and Methods for cell culture and ELISA protocols. M stands for medium-treated control. Each value is an average of three replicates, and the results are representative of at least two independent experiments.
Degradation of IκBα in J774 cells
By interacting with NF-κB, IκBs inhibit the translocation of NF-κB to the nucleus and thereby its binding to DNA (36,37). Therefore, the presence of NF-κB in nuclear extracts in general should correspond to the degradation of IκBs, although recent studies indicate that NF-κB activation could also occur without IκB degradation (38). We evaluated whether CpG DNA-mediated activation of NF-κB correlates with an enhanced and sustained degradation of IκBα in J774 cells. A western blot assay was performed on cytoplasmic extracts using specific antibodies against murine IκBα. As shown in Figure 5B, IκBα levels were only marginally affected by treatment with 1 for 30 min, corresponding to the minimal activation of NF-κB (Fig. 5A). After 1 h, IκBα had completely disappeared, but it reappeared after 3 h. In the case of treatment with immunomers 2 and 3, IκBα was rapidly degraded within 30 min and reappeared after 1 h.
Activation of the p38 MAPK pathway in J774 cells
CpG DNA is also known to activate the stress-activated p38 MAPK pathway (39,40). To evaluate whether immunomers 2 and 3 activate the p38 MAPK pathway similarly to 1, we measured the status of p38 phosphorylation in J774 cells following treatment with CpG DNAs for 30 min. All three CpG DNAs induced phosphorylation of p38 in J774 cells to similar extents (Fig. 6), although significant differences were observed in NF-κB activation.
Figure 6.
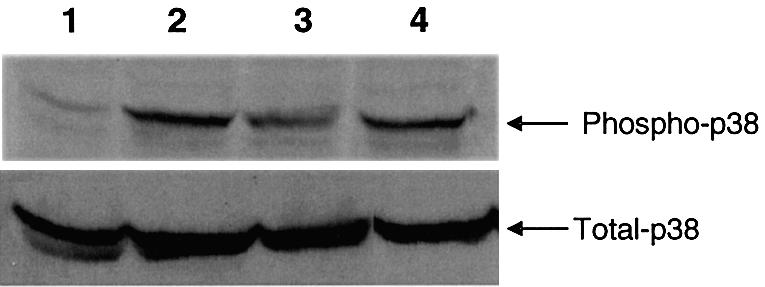
p38 phosphorylation in J774 macrophages. J774 cells were cultured, treated with PBS (lane 1) or 10 µg/ml of CpG DNAs 1 (lane 2), 2 (lane 3) or 3 (lane 4) for 0.5 h, and cell lysates were extracted and analyzed by western blotting using a phospho-p38 MAPK antibody as described in Materials and Methods. Total cellular p38 content is shown in the lower panel.
Consistent with rapid and strong activation of NF-κB by immunomers 2 and 3, higher levels of IL-12 and IL-6 induction were found in J774 cell cultures (Fig. 5C).
Effect of immunomers on cytokine induction in conalbumin-sensitized mouse spleen cell cultures
CpG DNA induces predominantly Th1-type immune responses. It has been shown that CpG DNA treatment reverses established antigen-induced Th2 responses to Th1-type responses (41,42). To assess the effects of treatment of immunomers on Th2 cytokines associated with allergic airway responses, we measured IL-5, IL-12 and IL-6 secreted in spleen cell cultures obtained from BALB/c mouse sensitized and challenged with conalbumin. In the absence of immunomer treatment, conalbumin-sensitized mouse spleen cells secreted markedly higher levels of IL-5, and low IL-12, suggesting predominantly a Th2 polarization (Table 3). When the allergen-primed spleen cells were treated with immunomers, a concentration-dependent decrease in IL-5 and increase in IL-12 secretion was observed. Table 3 shows the levels of IL-5, IL-12 and IL-6 induced following treatment with 1.0 µg/ml concentration of immunomers. These data suggest that immunomers not only induce Th1-type cytokine secretion but potently reverse antigen-induced Th2 responses. Sig nificantly, in these assays, immunomer 3 containing the CpR motif also induced lower levels of IL-6 compared with immunomer 2 containing the natural CpG motif.
Table 3. Cytokine induction by CpG DNA and immunomers in conalbumin-sensitized BALB/c mouse spleen cell cultures.
| CpG DNAa | IL-5 (pg/ml ± SD) | IL-12 (pg/ml ± SD) | IL-6 (pg/ml ± SD) |
|---|---|---|---|
| 1 | 567 ± 148 | 647 ± 102 | 1246 ± 109 |
| 2 | 131 ± 36 | 1660 ± 56 | 9567 ± 940 |
| 3 | 325 ± 7 | 1508 ± 98 | 4382 ± 694 |
| Medium | 1345 ± 437 | 45 ± 9 | 396 ± 12 |
aConalbumin-sensitized BALB/c mouse spleen cells were cultured, treated with different concentrations of CpG DNAs and the culture supernatants were assayed for the cytokines by ELISA as described in Materials and Methods. Data shown are for 1 µg/ml concentration of CpG DNA and immunomers. Each value is an average of three readings.
Antitumor activity in nude mice bearing human tumor xenografts
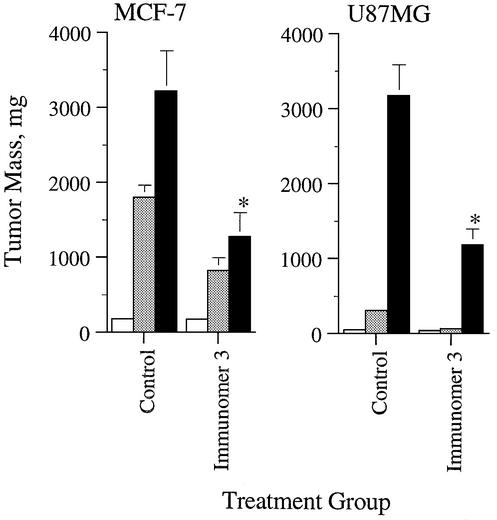
CpG DNAs have been shown to induce antitumor activity in vivo (28,29,43). Since the immunomer containing the synthetic CpR motif (3) induced potent immunostimulatory activity in cell culture assays, we evaluated its antitumor activity in vivo. Compound 3 was administered subcutaneously three times a week at a dose of 0.5 mg/kg to nude mice bearing MCF-7 human breast tumor or U87MG human glioblastoma xenografts that express wild-type p53. Growth of MCF-7 tumors was inhibited by 60% on day 15 compared with the saline control (Fig. 7). At the same dose, 3 inhibited growth of the glioblastoma by 63% on day 42 compared with the saline control (Fig. 7). At this dose, 3 did not cause any treatment-related toxicity in mice. There was no difference in body weight gains between treated animals and controls (data not shown). These preliminary antitumor studies suggest that immunomers containing synthetic CpR motifs exhibit potent antitumor activity in vivo.
Figure 7.
In vivo antitumor activity. Nude mice bearing MCF-7 human breast cancer (left panel) or U87MG glioblastoma (right panel) xenografts were treated with saline or immunomer 3 as specified in Materials and Methods. Data are shown for days 0, 9 and 15 for MCF-7, and for days 0, 28 and 42 for U87MG studies. Each group had six mice. Immunomer was given subcutaneously on every other day starting at day 0. *Represents a statistically significant value (P < 0.01).
DISCUSSION
Bacterial and synthetic CpG DNAs promote B cell proliferation, immunoglobulin production and the secretion of a number of cytokines, including IL-12, IFN-γ, IL-6 and TNF-α, from B cells, monocytes/macrophages, dendritic cells and natural killer cells. It has been shown that a receptor, TLR9, is engaged in the recognition of CpG DNA and subsequent initiation of the signaling cascade leading to the activation of the transcription factor NF-κB. In this study, we show that immunomers containing two accessible 5′-ends are more immunostimulaotry than a conventional CpG DNA, and the receptor recognizes a synthetic CpR motif in immunomers similarly to the natural CpG motif.
Our recent studies showed that covalent conjugation of molecules such as fluorescein at the 5′-end of a CpG DNA molecule abrogated the immunostimulatory activity, leaving uptake unaffected compared with the 3′-conjugate (26). Earlier studies of CpG DNAs containing multiple copies of CpG motifs showed no increased activity (25–27). From these results and those reported here, we conclude that the increased activity of 2 and 3 is not due to the presence of two copies of the CpG or CpR dinucleotide motif but to the presence of two 5′-accessible ends in the molecule. If this were not so, then similar cytokine induction would be expected if 1 were used at double the concentration of 2, giving the same concentration of CpG dinucleotide motifs. However, this is not the case. Additionally, immunomers linked 5′–5′ are inactive or much less active than 3′–3′-linked analogs or conventional CpG DNAs (25–27).
Compared with conventional CpG DNAs, a single administration of immunomer to mice resulted in significantly greater spleen enlargement, mononuclear cell expansion in spleen and serum IL-12 levels (44). A control immunomer lacking CpG was inactive, showing the need for this motif (data not shown). The greater systemic effects of immunomers could be due to (i) the presence of two accessible 5′-ends (25–29), and (ii) increased in vivo metabolic stability from a lack of 3′-ends for nuclease degradation (28,29,45,46).
Following recognition of CpG DNA by TLR9, a number of cellular events are initiated, including recruitment of MyD88, IRAK and TRAF6, and activation of IκB kinase, MAPK, the stress kinases N‐terminal c-Jun kinases JNK-1 and -2, and p38, leading to the activation of multiple transcription factors ATF-2, AP-1 and NF-κB. The transcription factors activated ultimately stimulate the synthesis of a number of cytokines and expression of co-stimulatory molecules. Like CpG DNA 1, immunomers activated NF-κB and p38 MAPK pathways in J774 cells. However, the kinetics of activation in the two cases are distinctly different. Generally, activation of NF-κB in J774 cells is more rapid and sustained with immunomers 2 and 3 than with 1. However, similar levels of p38 phosphorylation are observed with both immunomers and 1 after 1 h. Further kinetic studies are underway to distinguish any differences in the activation of the stress-induced MAPK pathway.
Only unmethylated CpG dinucleotide motifs have been shown to induce immunostimulatory effects. We and others have shown that the presence of a methyl group at the 5-position of cytosine abrogates immunostimulatory activity (12). Through the use of synthetic pyrimidines in place of cytidine, we showed that 5-hydroxyl and N4-ethyl substituents do not interfere with receptor recognition and immunostimulation (24). Similar studies with guanosine analogs showed the need for hydrogen bond donors at positions 1 and 2, and a hydrogen bond acceptor at position 6. However, the hydrogen bond-accepting N7 atom is not essential, and a 2′-deoxy-7-deaza-guanosine can be used in the synthetic immunostimulatory motif CpR (see Fig. 1 for structures) (24).
Here, we provide the first evidence that the CpR motif in immunomers is not only recognized by the receptor but induces a different cytokine secretion profile from that of the natural CpG motif. In both in vitro and in vivo studies, CpG immunomer 2 induced higher IL-12 and IL-6 than did conventional CpG DNA 1. Importantly, compared with 2, the CpR immunomer 3 consistently induced significantly lower levels of IL-6, but similar levels of IL-12 in vitro and in vivo. Elevated levels of IFN-γ expected with increased IL-12 expression were also observed [(27), and data not shown]. In allergen-sensitized mouse spleen cell cultures, both immunomers showed a potent inhibition of IL-5 secretion compared with CpG DNA 1. Immunomers showed similar IL-12 and IL-6 secretion profiles in Th2 polarized mouse spleen cell cultures to those found in normal mouse spleen cell cultures and in vivo. In addition to different cytokine secretion profiles, immunomer 3 showed a greater antigen-specific IgG2a/IgG1 antibody ratio compared with immunomer 2 (D.Wang and S.Agrawal, unpublished data), suggesting that the CpR motif is recognized by the receptor but results in distinct downstream effects compared with the normal CpG motif-containing immunomer. The kinetics of activation of NF-κB are similar with both immunomers, and it is not clear how they produce distinct cytokine expression profiles. Detailed mechanistic studies are required with different subsets of immune cells to distinguish the downstream events. Nonetheless, recognition of both natural CpG and synthetic CpR motifs suggests alternative NMRPs for the receptor, although the involvement of a related receptor cannot be ruled out. TLR3 that belongs to the same family specifically recognizes viral dsRNA and synthetic poly(I)·poly(C) (47). Greater IL-12 induction by immunomers could be of importance for cancer therapy (43,48) as well as for adjuvant action (49,50) with vaccines, allergens and monoclonal antibodies in therapy and prophylaxy. Induction of lower IL-6 and TNF-α levels [(27), and data not shown] by synthetic CpR immunomers may permit development of immunotherapeutic agents with reduced cytokine-related toxicities (51,52).
In conclusion, we have shown that immunomers induce rapid and sustained immunostimulation with distinct cytokine secretion profiles both in vitro and in vivo. Their increased activity results from two accessible 5′-ends and improved metabolic stability in vivo. For the first time, we have shown that the receptor recognizes a synthetic CpR motif and that alternative NMRPs produce different cytokine secretion profiles. The ability of the novel synthetic motif to induce immune stimulation resulted in potent antitumor activity in nude mice bearing MCF-7 human breast tumor and U87MG human glioblastoma xenografts. Additionally, no treatment-related toxcity was observed in mice at the dose studied. A number of other purine and pyrimidine analogs that are recognized by the receptor are under study currently for their distinct immunological properties. These studies will not only permit development of tailor-made immunotherapeutic agents for specific diseases but also illuminate receptor–CpG DNA recognition and signaling at the molecular level.
REFERENCES
- 1.Tokunaga T., Yamamoto,H., Shimada,S., Abe,H., Fukuda,T., Fujisawa,Y., Furutani,Y., Yano,O., Kataoka,T., Sudo,T., Makiguchi,N. and Suganuma,T. (1984) Antitumor activity of deoxyribonucleic acid fraction from Mycobacterium bovis BCG.I. Isolation, physicochemical characterization, and antitumor activity. J. Natl Cancer Inst., 72, 955–962. [PubMed]
- 2.Messina J.P., Gilkeson,G.S. and Pisetsky,D.S. (1991) Stimulation of in vitro murine lymphocyte proliferation by bacterial DNA. J. Immunol., 147, 1759–1764. [PubMed]
- 3.Krieg A.M., Yi,A.K., Matson,S., Waldschmidt,T.J., Bishop,G.A., Teasdale,R., Koretzky,G.A. and Klinman,D.M. (1995) CpG motifs in bacterial DNA trigger direct B-cell activation. Nature, 374, 546–549. [DOI] [PubMed]
- 4.Sato Y., Roman,M., Tighe,H., Lee,D., Corr,M., Nguyen,M.D., Silverman,G.J., Lotz,M., Carson,D.A. and Raz,E. (1996) Immunostimulatory DNA sequences necessary for effective intradermal gene immunization. Science, 273, 352–354. [DOI] [PubMed]
- 5.Yamamoto S., Kuramoto,E., Shimada,S. and Tokunaga,T. (1988) In vitro augmentation of natural killer cell activity and production of interferon-α/β and -γ with deoxyribonucleic acid fraction from Mycobacterium bovis BCG. Jpn J. Cancer Res., 79, 866–873. [DOI] [PMC free article] [PubMed]
- 6.Halpern M.D., Kurlander,R.J. and Pisetsky,D.S. (1996) Bacterial DNA induces murine interferon-γ production by stimulation of interleukin-12 and tumor necrosis factor-α. Cell. Immunol., 167, 72–78. [DOI] [PubMed]
- 7.Klinman D.M., Yi,A.K., Beaucage,S.L., Conover,J. and Krieg,A.M. (1996) CpG motifs present in bacteria DNA rapidly induce lymphocytes to secrete interleukin 6, interleukin 12 and interferon-γ. Proc. Natl Acad. Sci. USA, 93, 2879–2883. [DOI] [PMC free article] [PubMed]
- 8.Zhao Q., Temsamani,J., Zhou,R.Z. and Agrawal,S. (1997) Pattern and kinetics of cytokine production following administration of phosphorothioate oligonucleotides in mice. Antisense Nucleic Acid Drug Dev., 7, 495–502. [DOI] [PubMed]
- 9.Yamamoto S., Yamamoto,T., Kataoka,T., Kuramoto,E., Yano,O. and Tokunaga,T. (1992) Unique palindromic sequences in synthetic oligonucleotides are required to induce IFN and augment IFN-mediated natural killer activity. J. Immunol., 148, 4072–4076. [PubMed]
- 10.Liang H., Nishioka,Y., Reich,C.F., Pisetsky,D.S. and Lipsky,P.E. (1996) Activation of human B cells by phosphorothioate oligodeoxynucleotides. J. Clin. Invest., 98, 1119–1129. [DOI] [PMC free article] [PubMed]
- 11.vanUden J. and Raz,E. (2000) Introduction to immunostimulatory DNA sequences. Springer Semin. Immunopathol., 22, 1–9. [DOI] [PubMed]
- 12.Zhao Q., Temsamani,J., Iadarola,P.L., Jiang,Z. and Agrawal,S. (1996) Effect of different chemically modified oligodeoxynucleotides on immune stimulation. Biochem. Pharmacol., 51, 173–182. [DOI] [PubMed]
- 13.Kandimalla E.R., Yu,D. and Agrawal,S. (2002) Towards optimal design of second-generation immunomodulatory oligonucleotides. Curr. Opin. Mol. Ther., 4, 122–129. [PubMed]
- 14.Agrawal S. and Kandimalla,E.R. (2002) Medicinal chemistry and therapeutic potential of CpG DNA. Trends Mol. Med., 8, 114–121. [DOI] [PubMed]
- 15.Hemmi H., Takeuchi,O., Kawai,T., Kaisho,T., Sato,S., Sanjo,H., Matsumoto,M., Hoshino,K., Wagner,H., Takeda,K. and Akira,S. (2000) A Toll-like receptor recognizes bacterial DNA. Nature, 408, 740–745. [DOI] [PubMed]
- 16.Zhao Q., Yu,D. and Agrawal,S. (1999) Site of chemical modifications in CpG containing phosphorothioate oligodeoxynucleotide modulates its immunostimulatory activity. Bioorg. Med. Chem. Lett., 9, 3453–3458. [DOI] [PubMed]
- 17.Zhao Q., Yu,D. and Agrawal,S. (2000) Immunostimulatory activity of CpG containing phosphorothioate oligodeoxynucleotide is modulated by modification of a single deoxynucleoside. Bioorg. Med. Chem. Lett., 10, 1051–1054. [DOI] [PubMed]
- 18.Yu D., Kandimalla,E.R., Zhao,Q., Cong,Y. and Agrawal,S. (2002) Immunostimulatory properties of phosphorothioate CpG DNA containing both 3′–5′- and 2′–5′-internucleotide linkages. Nucleic Acids Res., 30, 1613–1619. [DOI] [PMC free article] [PubMed]
- 19.Agrawal S. and Kandimalla,E.R. (2001) Antisense and/or immunostimulatory oligonucleotide therapeutics. Curr. Cancer Drug Targets, 1, 197–209. [DOI] [PubMed]
- 20.Yu D., Kandimalla,E.R., Zhao,Q., Cong,Y. and Agrawal,S. (2001) Immunostimulatory activity of CpG oligonucleotides containing non-ionic methylphosphonate linkages. Bioorg. Med. Chem., 9, 2803–2808. [DOI] [PubMed]
- 21.Yu D., Kandimalla,E.R., Zhao,Q., Cong,Y. and Agrawal,S. (2001) Modulation of immunostimulatory activity of CpG oligonucleotides by site-specific deletion of nucleobases. Bioorg. Med. Chem. Lett., 11, 2263–2267. [DOI] [PubMed]
- 22.Yu D., Kandimalla,E.R., Zhao,Q., Bhagat,L., Cong,Y. and Agrawal,S. (2003) Requirement of nucleotide bases proximal to a CpG-motif for immunostimulatory activity of synthetic oligonucleotides. Bioorg. Med. Chem., 11, 459–464. [DOI] [PubMed]
- 23.Yu D., Kandimalla,E.R., Cong,Y., Tang,J., Tang,J.Y., Zhao,Q. and Agrawal,S. (2002) Design, synthesis, and immunostimulatory properties of CpG DNAs containing alkyl-linker substitutions: role of nucleosides in the flanking sequences. J. Med. Chem., 45, 4540–4548. [DOI] [PubMed]
- 24.Kandimalla E.R., Yu,D., Zhao,Q. and Agrawal,S. (2001) Effect of chemical modifications of cytosine and guanine in a CpG-motif of oligonucleotides: structure–immunostimulatory activity relationships. Bioorg. Med. Chem., 9, 807–813. [DOI] [PubMed]
- 25.Yu D., Zhao,Q., Kandimalla,E.R. and Agrawal,S. (2000) Accessible 5′-end of CpG-containing phosphorothioate oligodeoxynucleotides is essential for immunostimulatory activity. Bioorg. Med. Chem. Lett., 10, 2585–2588. [DOI] [PubMed]
- 26.Kandimalla E.R., Bhagat,L., Yu,D., Cong,Y., Tang,J. and Agrawal,S. (2002) Conjugation of ligands at the 5′-end of CpG DNA affects immunostimulatory activity. Bioconjug. Chem., 13, 966–974. [DOI] [PubMed]
- 27.Yu D., Kandimalla,E.R., Bhagat,L., Tang,J.Y., Cong,Y., Tang,J. and Agrawal,S. (2002) ‘Immunomers’—novel 3′–3′-linked CpG oligodeoxyribonucleotides as potent immunomodulatory agents. Nucleic Acids Res., 30, 4460–4469. [DOI] [PMC free article] [PubMed]
- 28.Yu D., Zhu,F.G., Bhagat,L., Wang,H., Kandimalla,E.R., Zhang,R. and Agrawal,S. (2002) Potent CpG oligonucleotides containing phosphodiester linkages: in vitro and in vivo immunostimulatory properties. Biochem. Biophys. Res. Commun., 297, 83–90. [DOI] [PubMed]
- 29.Bhagat L., Zhu,F.G., Yu,D., Tang,J., Wang,H., Kandimalla,E.R., Zhang,R. and Agrawal,S. (2003) CpG penta- and hexadeoxyribonucleotides as potent immunomodulatory agents. Biochem. Biophys. Res. Commun., 300, 853–861. [DOI] [PubMed]
- 30.Iyer R.P., Egan,W., Regan,J.B. and Beaucage,S.L. (1990) 3H-1,2 benzodithiole-3-one1,1-dioxide as an improved sulfurizing reagent in the solid-phase synthesis of oligodeoxyribonucleoside phosphorothioates. J. Am. Chem. Soc., 112, 1253–1254.
- 31.Puglisi J.D. and Tinoco,I.,Jr (1989) Absorbance melting curves of RNA. Methods Enzymol., 180, 304–325. [DOI] [PubMed]
- 32.Branda R.F., Moore,A.L., Mathews,L., McCormack,J.J. and Zon,G. (1993) Immune stimulation by an antisense oligomer complementrary to the rev gene of HIV-1. Biochem. Pharmacol., 45, 2037–2043. [DOI] [PubMed]
- 33.Wang H., Nan,L., Yu,D., Agrawal,S. and Zhang,R. (2001) Antisense anti-MDM2 oligonucleotides as a novel therapeutic approach to human breast cancer: in vitro and in vivo activities and mechanisms. Clin. Cancer Res., 7, 3613–3624. [PubMed]
- 34.Prasad G., Wang,H., Agrawal,S. and Zhang,R. (2002) Antisense anti-MDM2 oligonucleotides as a novel approach to the treatment of glioblastoma multiforme. Anticancer Res., 22, 107–116. [PubMed]
- 35.Stacey K.J., Sweet,M.J. and Hume,D.A. (1996) Macrophages ingest and are activated by bacterial DNA. J. Immunol., 157, 2116–2122. [PubMed]
- 36.Henkel T., Machleidt,T., Alkalay,I., Kronke,M., Ben-Neriah,Y. and Baeuerle,P.A. (1993) Rapid proteolysis of IκB-α is necessary for activation of transcription factor NF-κB. Nature, 365, 182–185. [DOI] [PubMed]
- 37.Suyang H., Phillips,R., Douglas,I. and Ghosh,S. (1996) Role of unphosphorylated, newly synthesized IκBβ in persistent activation of NF-κB. Mol. Cell. Biol., 16, 5444–5449. [DOI] [PMC free article] [PubMed]
- 38.Imbert V., Rupec,R.A., Livolsi,A., Pahl,H.L., Traenckner,E.B., Mueller-Dieckmann,C., Farahifar,D., Rossi,B., Auberger,P., Baeuerle,P.A. and Peyron,J.F. (1996) Tyrosine phosphorylation of IκB-α activates NF-κB without proteolytic degradation of IκB-α. Cell, 86, 787–798. [DOI] [PubMed]
- 39.Yi A. and Krieg,A.M. (1998) Rapid induction of mitogen-activated protein kinases by immune stimulatory CpG DNA. J. Immunol., 161, 4493–4497. [PubMed]
- 40.Choudhury B.K., Wild,J.S., Alam,R., Klinman,D.M., Boldogh,I., Dharajiya,N., Mileski,W.J. and Sur,S. (2002) In vivo role of p38 mitogen-activated protein kinase in mediating the anti-inflammatory effects of CpG oligodeoxynucleotide in murine asthma. J. Immunol., 169, 5955–5961. [DOI] [PubMed]
- 41.Serebrisky D., Teper,A.A., Huang,C.K., Lee,S.Y., Zhang,T.F., Schofield,B.H., Kattan,M., Sampson,H.A. and Li,X.M. (2000) CpG oligodeoxynucleotides can reverse Th2-associated allergic airway responses and alter the B7.1/B7.2 expression in a murine model of asthma. J. Immunol. 165, 5906–5912. [DOI] [PubMed]
- 42.Kitagaki K., Jain,V.V., Businga,T.R., Hussain,I. and Kline,J.N. (2002) Immunomodulatory effects of CpG oligodeoxynucleotides on established Th2 responses. Clin. Diagn. Lab. Immunol., 9, 1260–1269. [DOI] [PMC free article] [PubMed]
- 43.Carpentier A.F., Auf,G. and Delattre,J.-Y. (2003) CpG-oligonucleotides for cancer immunotherapy: review of the literature and potential applications in malignant glioma. Front. Biosci., 8, e115–127. [DOI] [PubMed]
- 44.Davila E., Velez,M.G., Heppelmann,C.J. and Celis,E. (2002) Creating space: an antigen-independent, CpG-induced peripheral expansion of naive and memory T lymphocytes in a full T-cell compartment. Blood, 100, 2537–2545. [DOI] [PubMed]
- 45.Kandimalla E.R., Agrawal,S., Venkataraman,S. and Sasisekharan,V. (1995) Single strand targeted triplex formation: parallel-stranded DNA hairpin duplexes for targeting pyrimidine strands. J. Am. Chem. Soc., 117, 6416–6417.
- 46.Chaix C., Iyer,R.P. and Agrawal,S. (1996) 3′–3′-Linked oligonucleotides synthesis and stability studies. Bioorg. Med. Chem. Lett., 6, 827–832. [DOI] [PubMed]
- 47.Alexopoulou L., Holt,A.C., Medzhitov,R. and Flavell,R.A. (2001) Recognition of double-stranded RNA and activation of NF-κB by Toll-like receptor 3. Nature, 413, 732–738. [DOI] [PubMed]
- 48.Decker T. and Peschel,C. (2001) Effect of immunostimulatory CpG-oligonucleotides in chronic lymphocytic leukemia B cells. Leuk. Lymphoma, 42, 301–307. [DOI] [PubMed]
- 49.Moingeon P., Haensler,J. and Lindberg,A. (2001) Towards the rational design of Th1 adjuvants. Vaccine, 19, 4363–4372. [DOI] [PubMed]
- 50.Leitner W.W., Hammerl,P. and Thalhamer,J. (2001) Nucleic acid for the treatment of cancer: genetic vaccines and DNA adjuvants. Curr. Pharm. Des., 7, 1641–1667. [DOI] [PubMed]
- 51.Klinman D.M., Kamstrup,S., Verthelyi,D., Gursel,I., Ishii,K.J., Takeshita,F. and Gursel,M. (2000) Activation of the innate immune system by CpG oligodeoxynucleotides: immunoprotective activity and safety. Springer Semin. Immunopathol., 22, 173–183. [DOI] [PubMed]
- 52.Wagner H., Lipford,G.B. and Hacker,H. (2000) The role of immunostimulatory CpG-DNA in septic shock. Springer Semin. Immunopathol., 22, 167–172. [DOI] [PubMed]