Abstract
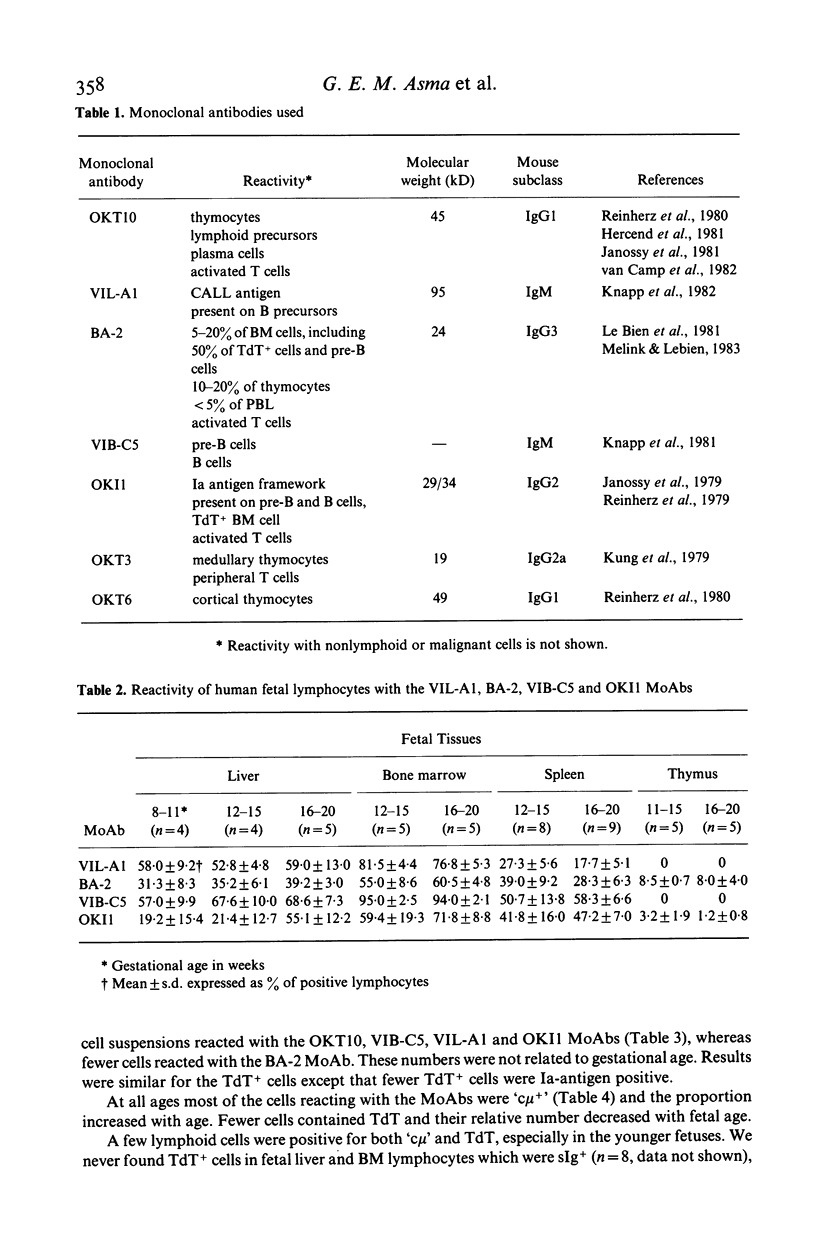
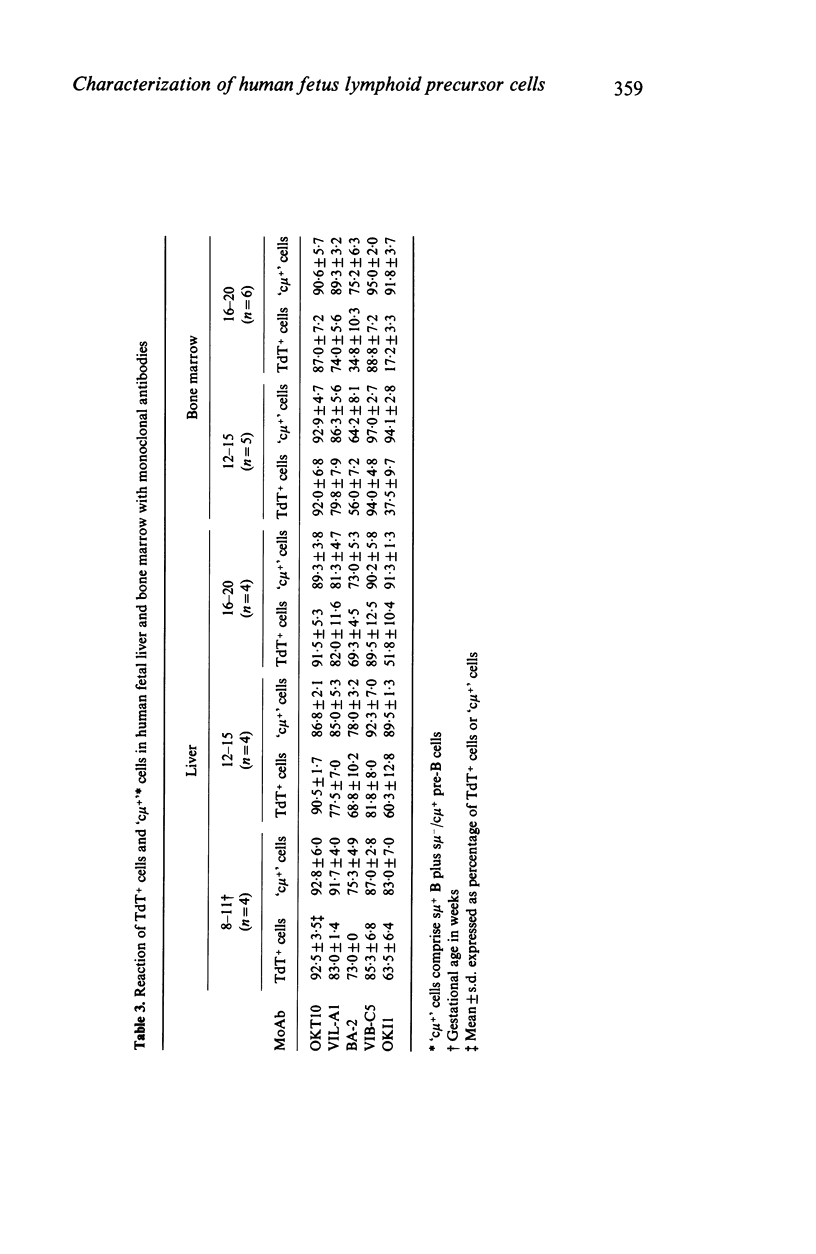
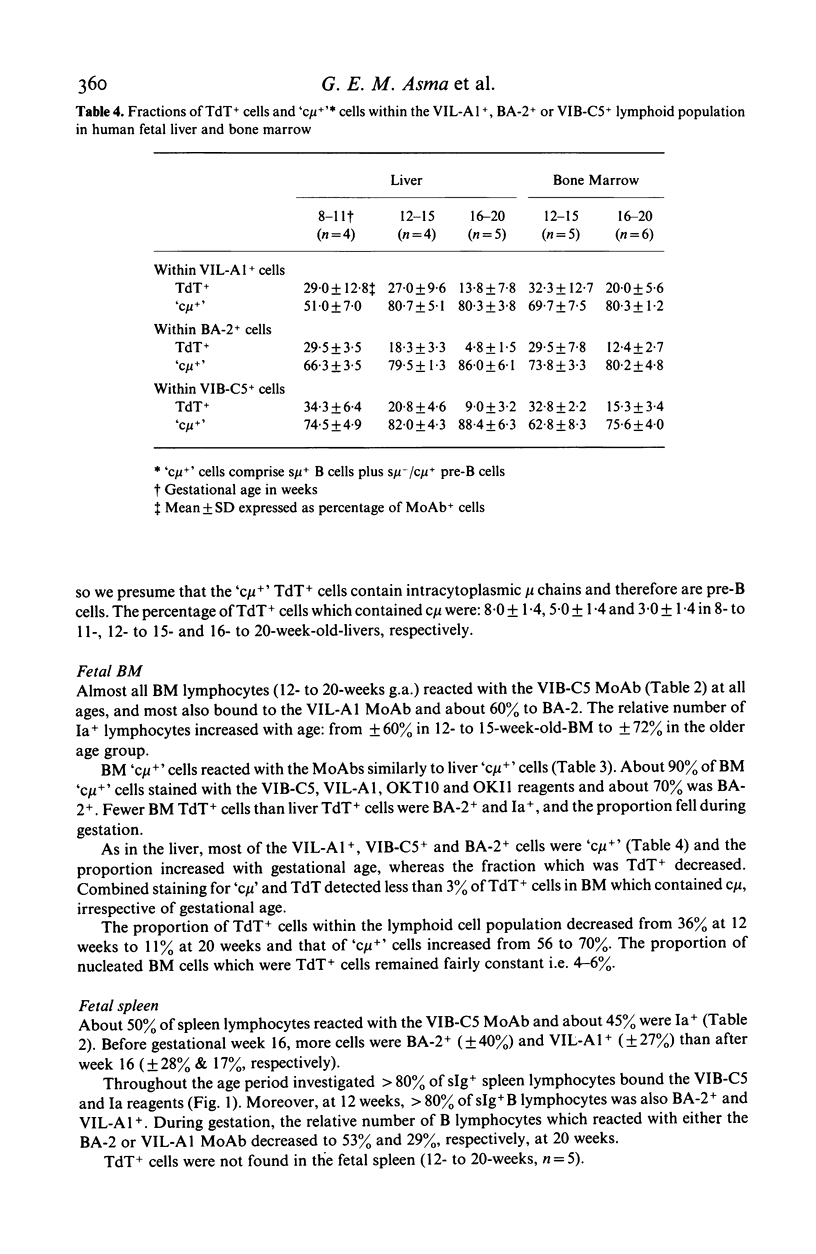
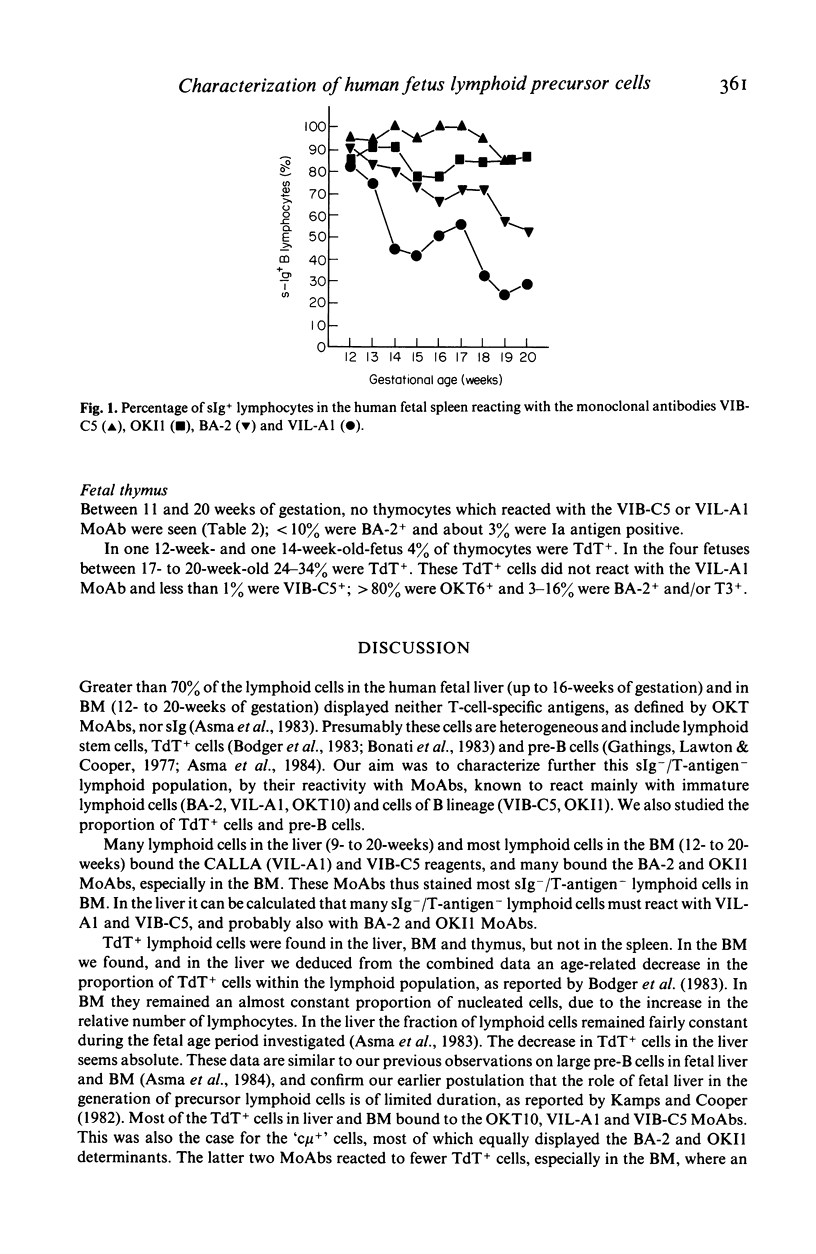
Monoclonal antibodies (MoAbs) directed primarily against immature lymphoid cells (VIL-A1, BA-2, OKT10) or recognizing antigens associated with the B cell lineage (VIB-C5, OKI1) were used for the identification of lymphoid cells in liver, bone marrow, spleen and thymus of human fetuses between 8 and 20 weeks of gestational age. Many lymphocytes in liver, bone marrow and spleen reacted with the MoAbs used. In the fetal thymus, however, cells did not bind to the VIL-A1 and VIB-C5 MoAbs and only a few cells were BA-2+ or OKI1+. In the liver and bone marrow the VIL-A1, VIB-C5 and BA-2 MoAbs reacted almost exclusively with terminal deoxynucleotidyl transferase (TdT) containing cells, pre-B and B cells. TdT+ cells were present in liver, bone marrow and thymus, but not in the spleen. In liver and bone marrow the relative numbers of TdT+ cells decreased during gestation, in the thymus they increased. The antigenic make-up of the TdT+ cells in liver and bone marrow was comparable to that of pre-B and B cells in these organs: most of them reacted with VIL-A1, VIB-C5 and OKT10 MoAbs and many were BA-2+ and OKI1+. TdT+ cells in liver and bone marrow did not bind to T-cell-markers, i.e. OKT6 and WT-1. A few lymphoid cells in these organs contained TdT and mu heavy chains. TdT+ cells in the thymus had a completely different phenotype: most of them were OKT6+ and they did not react with the VIL-A1 and VIB-C5 MoAbs. These findings suggest that TdT+ cells in fetal liver and bone marrow are precursors of the B cell lineage, whereas those in the thymus probably belong to the T cell lineage. In the fetal spleen almost all B cells displayed the VIB-C5 and OKI1 antigens. At 12 weeks of gestation greater than 80% of splenic B cells were also VIL-A1+ and BA-2+; with ongoing gestation far less B cells in spleen expressed these antigens, however, indicating that these B cells are more mature than those in fetal liver and bone marrow, but still less mature than the B cells in postnatal blood and bone marrow, which do not display the VIL-A1 and BA-2 markers. These findings suggest that some further maturation of B cell stages takes place in the spleen during human fetal life.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asma G. E., Langlois van den Bergh R., Vossen J. M. Development of pre-B and B lymphocytes in the human fetus. Clin Exp Immunol. 1984 May;56(2):407–414. [PMC free article] [PubMed] [Google Scholar]
- Asma G. E., Van den Bergh R. L., Vossen J. M. Use of monoclonal antibodies in a study of the development of T lymphocytes in the human fetus. Clin Exp Immunol. 1983 Aug;53(2):429–436. [PMC free article] [PubMed] [Google Scholar]
- Barr R. D., Sarin P. S., Perry S. M. Terminal transferase in human bone-marrow lymphocytes. Lancet. 1976 Mar 6;1(7958):508–509. doi: 10.1016/s0140-6736(76)90295-6. [DOI] [PubMed] [Google Scholar]
- Bodger M. P., Janossy G., Bollum F. J., Burford G. D., Hoffbrand A. V. The ontogeny of terminal deoxynucleotidyl transferase positive cells in the human fetus. Blood. 1983 Jun;61(6):1125–1131. [PubMed] [Google Scholar]
- Bollum F. J. Terminal deoxynucleotidyl transferase as a hematopoietic cell marker. Blood. 1979 Dec;54(6):1203–1215. [PubMed] [Google Scholar]
- Bollum F. J. Terminal deoxynucleotidyl transferase: biological studies. Adv Enzymol Relat Areas Mol Biol. 1978;47:347–374. doi: 10.1002/9780470122921.ch6. [DOI] [PubMed] [Google Scholar]
- Bonati A., Casoli C., Starcich B., Buscaglia M. Terminal deoxynucleotidyl transferase (TdT) in human foetuses. An immunofluorescent and biochemical study. Scand J Haematol. 1983 Nov;31(5):447–453. doi: 10.1111/j.1600-0609.1983.tb01541.x. [DOI] [PubMed] [Google Scholar]
- Bonati A., Delia D., Starcich B., Buscaglia M. Phenotype of the terminal transferase-positive cells in human foetal liver and bone-marrow: analysis with monoclonal antibodies. Scand J Haematol. 1984 Nov;33(5):418–424. doi: 10.1111/j.1600-0609.1984.tb00719.x. [DOI] [PubMed] [Google Scholar]
- Brashem C. J., Kersey J. H., Bollum F. J., LeBien T. W. Ontogenic studies of lymphoid progenitor cells in human bone marrow. Exp Hematol. 1982 Nov;10(10):886–892. [PubMed] [Google Scholar]
- Gathings W. E., Lawton A. R., Cooper M. D. Immunofluorescent studies of the development of pre-B cells, B lymphocytes and immunoglobulin isotype diversity in humans. Eur J Immunol. 1977 Nov;7(11):804–810. doi: 10.1002/eji.1830071112. [DOI] [PubMed] [Google Scholar]
- Greaves M., Delia D., Janossy G., Rapson N., Chessells J., Woods M., Prentice G. Acute lymphoblastic leukaemia associated antigen. IV. Expression on non-leukaemic 'lymphoid' cells. Leuk Res. 1980;4(1):15–32. doi: 10.1016/0145-2126(80)90044-2. [DOI] [PubMed] [Google Scholar]
- Hercend T., Ritz J., Schlossman S. F., Reinherz E. L. Comparative expression of T9, T10, and Ia antigens on activated human T cell subsets. Hum Immunol. 1981 Nov;3(3):247–259. doi: 10.1016/0198-8859(81)90021-5. [DOI] [PubMed] [Google Scholar]
- Janossy G., Bollum F. J., Bradstock K. F., McMichael A., Rapson N., Greaves M. F. Terminal transferase-positive human bone marrow cells exhibit the antigenic phenotype of common acute lymphoblastic leukemia. J Immunol. 1979 Oct;123(4):1525–1529. [PubMed] [Google Scholar]
- Janossy G., Thomas J. A., Bollum F. J., Granger S., Pizzolo G., Bradstock K. F., Wong L., McMichael A., Ganeshaguru K., Hoffbrand A. V. The human thymic microenvironment: an immunohistologic study. J Immunol. 1980 Jul;125(1):202–212. [PubMed] [Google Scholar]
- Janossy G., Tidman N., Papageorgiou E. S., Kung P. C., Goldstein G. Distribution of t lymphocyte subsets in the human bone marrow and thymus: an analysis with monoclonal antibodies. J Immunol. 1981 Apr;126(4):1608–1613. [PubMed] [Google Scholar]
- Kamps W. A., Cooper M. D. Microenvironmental studies of pre-B and B cell development in human and mouse fetuses. J Immunol. 1982 Aug;129(2):526–531. [PubMed] [Google Scholar]
- Kersey J. H., LeBien T. W., Abramson C. S., Newman R., Sutherland R., Greaves M. P-24: a human leukemia-associated and lymphohemopoietic progenitor cell surface structure identified with monoclonal antibody. J Exp Med. 1981 Mar 1;153(3):726–731. doi: 10.1084/jem.153.3.726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp W., Majdic O., Bettelheim P., Liszka K. VIL-A1, a monoclonal antibody reactive with common acute lymphatic leukemia cells. Leuk Res. 1982;6(2):137–147. doi: 10.1016/0145-2126(82)90019-4. [DOI] [PubMed] [Google Scholar]
- Kung P., Goldstein G., Reinherz E. L., Schlossman S. F. Monoclonal antibodies defining distinctive human T cell surface antigens. Science. 1979 Oct 19;206(4416):347–349. doi: 10.1126/science.314668. [DOI] [PubMed] [Google Scholar]
- Melink G. B., LeBien T. W. Construction of an antigenic map for human B-cell precursors. J Clin Immunol. 1983 Jul;3(3):260–267. doi: 10.1007/BF00915350. [DOI] [PubMed] [Google Scholar]
- Pearl E. R., Vogler L. B., Okos A. J., Crist W. M., Lawton A. R., 3rd, Cooper M. D. B lymphocyte precursors in human bone marrow: an analysis of normal individuals and patients with antibody-deficiency states. J Immunol. 1978 Apr;120(4):1169–1175. [PubMed] [Google Scholar]
- Reinherz E. L., Kung P. C., Goldstein G., Levey R. H., Schlossman S. F. Discrete stages of human intrathymic differentiation: analysis of normal thymocytes and leukemic lymphoblasts of T-cell lineage. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1588–1592. doi: 10.1073/pnas.77.3.1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinherz E. L., Kung P. C., Pesando J. M., Ritz J., Goldstein G., Schlossman S. F. Ia determinants on human T-cell subsets defined by monoclonal antibody. Activation stimuli required for expression. J Exp Med. 1979 Dec 1;150(6):1472–1482. doi: 10.1084/jem.150.6.1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverstone A. E., Cantor H., Goldstein G., Baltimore D. Terminal deoxynucleotidyl transferase is found in prothymocytes. J Exp Med. 1976 Aug 1;144(2):543–548. doi: 10.1084/jem.144.2.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vossen J. M. Membrane-associated immunoglobulin determinants on bone marrow and blood lymphocytes in the pediatric age group and on fetal tissues. Ann N Y Acad Sci. 1975 Jun 30;254:262–279. doi: 10.1111/j.1749-6632.1975.tb29176.x. [DOI] [PubMed] [Google Scholar]


