Abstract
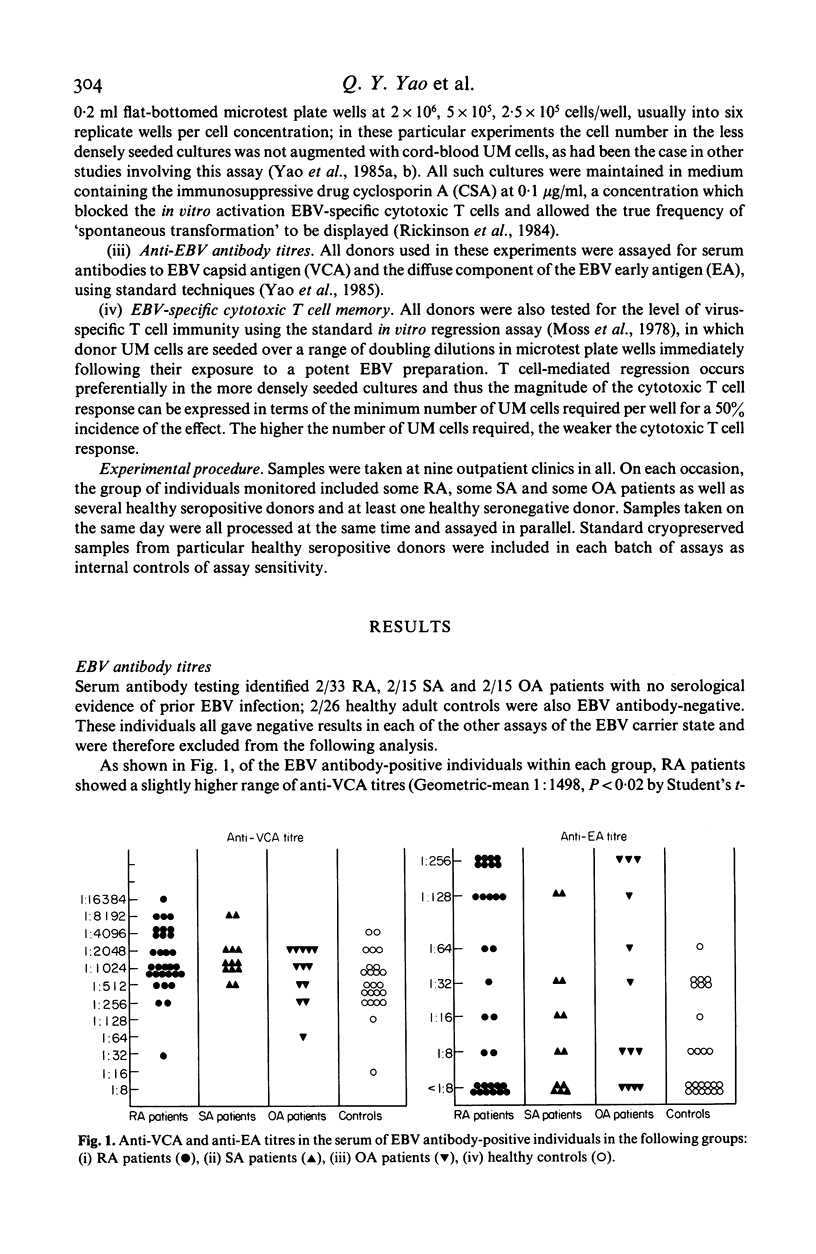
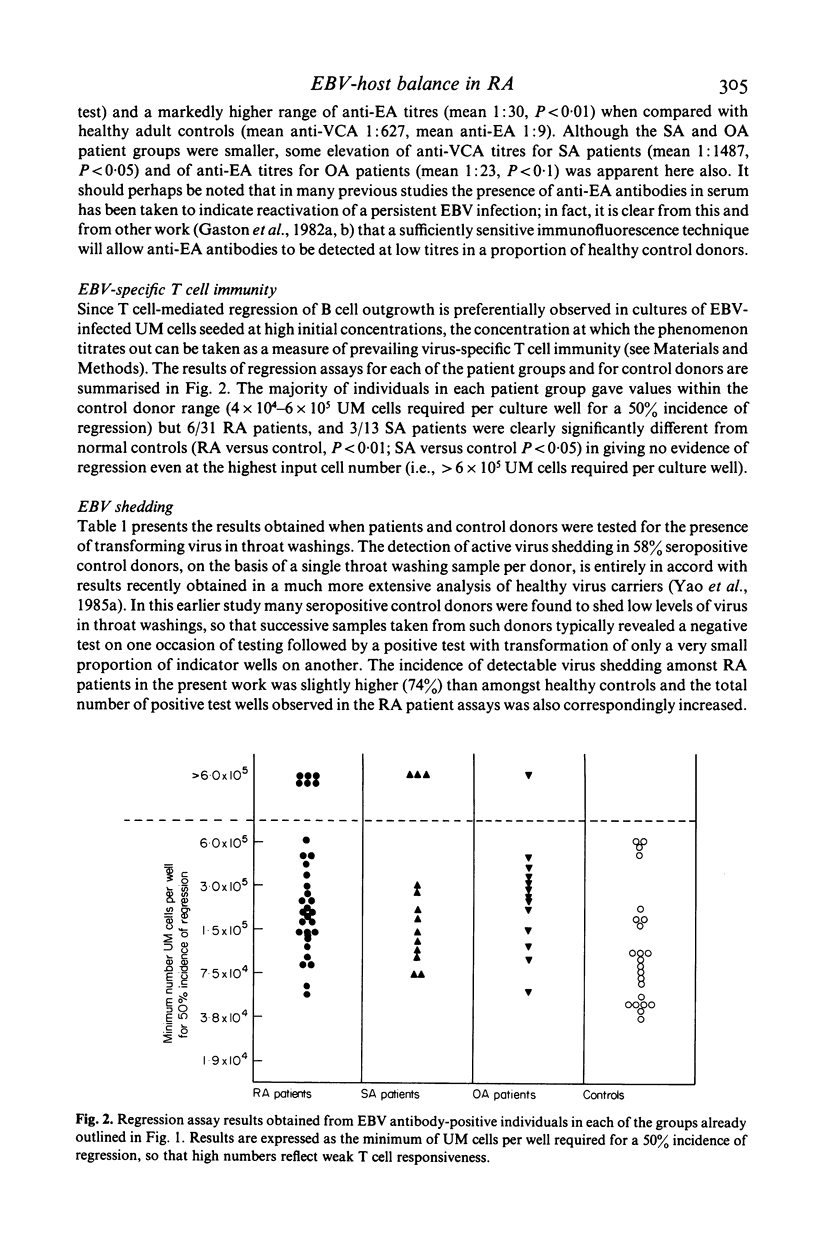
Rheumatoid arthritis (RA), seronegative spondyloarthropathy (SA) and osteoarthritis (OA) patients receiving no steroid or disease-modifying therapy have been monitored, along with healthy controls, for their prevailing level of Epstein-Barr virus (EBV) infection using four independent indices of the EBV-host balance, levels of virus shedding in throat washings as measured by a cord-blood transformation assay of improved sensitivity, frequency of virus-infected B cells in the circulating blood as measured by the rate of 'spontaneous' transformation in limiting dilution cultures, antibody titres to viral antigens, and virus-specific cytotoxic T cell responsiveness as measured in the in vitro regression assay. All four parameters indicated significant disturbance of the virus-host balance accompanying RA, the range of values exhibited by RA patients as a group in each case extending beyond the normal control range in the direction of more active infection. However, observations with SA and OA patients suggested that such a disturbance may not be RA-specific.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aitcheson C. T., Wilson G. L., Ferrell P. B., Tan E. M. Frequency of transforming Epstein-Barr virus in oropharyngeal secretions of rheumatoid arthritis patients. Intervirology. 1983;19(3):135–143. doi: 10.1159/000149348. [DOI] [PubMed] [Google Scholar]
- Alspaugh M. A., Henle G., Lennette E. T., Henle W. Elevated levels of antibodies to Epstein-Barr virus antigens in sera and synovial fluids of patients with rheumatoid arthritis. J Clin Invest. 1981 Apr;67(4):1134–1140. doi: 10.1172/JCI110127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alspaugh M. A., Jensen F. C., Rabin H., Tan E. M. Lymphocytes transformed by Epstein-Barr virus. Induction of nuclear antigen reactive with antibody in rheumatoid arthritis. J Exp Med. 1978 Apr 1;147(4):1018–1027. doi: 10.1084/jem.147.4.1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson U., Britton S., De Ley M., Bird G. Evidence for the ontogenic precedence of suppressor T cell functions in the human neonate. Eur J Immunol. 1983 Jan;13(1):6–13. doi: 10.1002/eji.1830130104. [DOI] [PubMed] [Google Scholar]
- Aslpaugh M. A., Tan E. M. Serum antibody in rheumatoid arthritis reactive with a cell-associated antigen. Demonstration by precipitation and immunofluorescence. Arthritis Rheum. 1976 Jul-Aug;19(4):711–719. doi: 10.1002/1529-0131(197607/08)19:4<711::aid-art1780190409>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Bardwick P. A., Bluestein H. G., Zvaifler N. J., Depper J. M., Seegmiller J. E. Altered regulation of Epstein-Barr virus induced lymphoblast proliferation in rheumatoid arthritis lymphoid cells. Arthritis Rheum. 1980 Jun;23(6):626–632. doi: 10.1002/art.1780230603. [DOI] [PubMed] [Google Scholar]
- Billings P. B., Hoch S. O., White P. J., Carson D. A., Vaughan J. H. Antibodies to the Epstein-Barr virus nuclear antigen and to rheumatoid arthritis nuclear antigen identify the same polypeptide. Proc Natl Acad Sci U S A. 1983 Dec;80(23):7104–7108. doi: 10.1073/pnas.80.23.7104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catalano M. A., Carson D. A., Niederman J. C., Feorino P., Vaughan J. H. Antibody to the rheumatoid arthritis nuclear antigen. Its relationship to in vivo Epstein-Barr virus infection. J Clin Invest. 1980 May;65(5):1238–1242. doi: 10.1172/JCI109779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catalano M. A., Carson D. A., Slovin S. F., Richman D. D., Vaughan J. H. Antibodies to Epstein-Barr virus-determined antigens in normal subjects and in patients with seropositive rheumatoid arthritis. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5825–5828. doi: 10.1073/pnas.76.11.5825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig J. C., Haire M., Millar J. H., Fraser K. B., Merrett J. D. Immunological control of Epstein-Barr virus-transformed lymphocytes in multiple sclerosis. Clin Immunol Immunopathol. 1983 Oct;29(1):86–93. doi: 10.1016/0090-1229(83)90009-0. [DOI] [PubMed] [Google Scholar]
- Crawford D. H., Edwards J. M., Sweny P., Hoffbrand A. V., Janossy G. Studies on long-term T-cell-mediated immunity to Epstein-BArr virus in immunosuppressed renal allograft recipients. Int J Cancer. 1981 Dec;28(6):705–709. doi: 10.1002/ijc.2910280608. [DOI] [PubMed] [Google Scholar]
- Depper J. M., Bluestein H. G., Zvaifler N. J. Impaired regulation of Epstein-Barr virus-induced lymphocyte proliferation in rheumatoid arthritis is due to a T cell defect. J Immunol. 1981 Nov;127(5):1899–1902. [PubMed] [Google Scholar]
- Depper J. M., Zvaifler N. J. Epstein-Barr virus. Its relationship to the pathogenesis of rheumatoid arthritis. Arthritis Rheum. 1981 Jun;24(6):755–761. doi: 10.1002/art.1780240601. [DOI] [PubMed] [Google Scholar]
- Ferrell P. B., Aitcheson C. T., Pearson G. R., Tan E. M. Seroepidemiological study of relationships between Epstein-Barr virus and rheumatoid arthritis. J Clin Invest. 1981 Mar;67(3):681–687. doi: 10.1172/JCI110083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaston J. S., Rickinson A. B., Epstein M. A. Epstein-Barr virus-specific cytotoxic T cell responses in rheumatoid arthritis patients. Rheumatol Int. 1982;2(4):155–159. doi: 10.1007/BF00286137. [DOI] [PubMed] [Google Scholar]
- Gaston J. S., Rickinson A. B., Epstein M. A. Epstein-Barr-virus-specific T-cell memory in renal-allograft recipients under long-term immunosuppression. Lancet. 1982 Apr 24;1(8278):923–925. doi: 10.1016/s0140-6736(82)91930-4. [DOI] [PubMed] [Google Scholar]
- Hasler F., Bluestein H. G., Zvaifler N. J., Epstein L. B. Analysis of the defects responsible for the impaired regulation of EBV-induced B cell proliferation by rheumatoid arthritis lymphocytes. II. Role of monocytes and the increased sensitivity of rheumatoid arthritis lymphocytes to prostaglandin E. J Immunol. 1983 Aug;131(2):768–772. [PubMed] [Google Scholar]
- Hasler F., Bluestein H. G., Zvaifler N. J., Epstein L. B. Analysis of the defects responsible for the impaired regulation of Epstein-Barr virus-induced B cell proliferation by rheumatoid arthritis lymphocytes. I. Diminished gamma interferon production in response to autologous stimulation. J Exp Med. 1983 Jan 1;157(1):173–188. doi: 10.1084/jem.157.1.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henle W., Henle G. Epstein-Barr virus-specific serology in immunologically compromised individuals. Cancer Res. 1981 Nov;41(11 Pt 1):4222–4225. [PubMed] [Google Scholar]
- Hennessy K., Kieff E. One of two Epstein-Barr virus nuclear antigens contains a glycine-alanine copolymer domain. Proc Natl Acad Sci U S A. 1983 Sep;80(18):5665–5669. doi: 10.1073/pnas.80.18.5665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss D. J., Klestov A., Burrows S., Kane R. G. A comparison of Epstein-Barr virus-specific T-cell immunity in rheumatoid arthritis and osteoarthritis patients. Aust J Exp Biol Med Sci. 1983 Oct;61(Pt 5):509–516. doi: 10.1038/icb.1983.48. [DOI] [PubMed] [Google Scholar]
- Moss D. J., Rickinson A. B., Pope J. H. Long-term T-cell-mediated immunity to Epstein-Barr virus in man. I. Complete regression of virus-induced transformation in cultures of seropositive donor leukocytes. Int J Cancer. 1978 Dec;22(6):662–668. doi: 10.1002/ijc.2910220604. [DOI] [PubMed] [Google Scholar]
- Rickinson A. B., Rowe M., Hart I. J., Yao Q. Y., Henderson L. E., Rabin H., Epstein M. A. T-cell-mediated regression of "spontaneous" and of Epstein-Barr virus-induced B-cell transformation in vitro: studies with cyclosporin A. Cell Immunol. 1984 Sep;87(2):646–658. doi: 10.1016/0008-8749(84)90032-7. [DOI] [PubMed] [Google Scholar]
- Sumaya C. V., Myers L. W., Ellison G. W. Epstein-Barr virus antibodies in multiple sclerosis. Arch Neurol. 1980 Feb;37(2):94–96. doi: 10.1001/archneur.1980.00500510052009. [DOI] [PubMed] [Google Scholar]
- Thorley-Lawson D. A. The transformation of adult but not newborn human lymphocytes by Epstein Barr virus and phytohemagglutinin is inhibited by interferon: the early suppression by T cells of Epstein Barr infection is mediated by interferon. J Immunol. 1981 Mar;126(3):829–833. [PubMed] [Google Scholar]
- Tosato G., Steinberg A. D., Blaese R. M. Defective EBV-specific suppressor T-cell function in rheumatoid arthritis. N Engl J Med. 1981 Nov 19;305(21):1238–1243. doi: 10.1056/NEJM198111193052102. [DOI] [PubMed] [Google Scholar]
- Tosato G., Steinberg A. D., Yarchoan R., Heilman C. A., Pike S. E., De Seau V., Blaese R. M. Abnormally elevated frequency of Epstein-Barr virus-infected B cells in the blood of patients with rheumatoid arthritis. J Clin Invest. 1984 Jun;73(6):1789–1795. doi: 10.1172/JCI111388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venables P. J., Roffe L. M., Erhardt C. C., Maini R. N., Edwards J. M., Porter A. D. Titers of antibodies to RANA in rheumatoid arthritis and normal sera. Relationship to Epstein-Barr virus infection. Arthritis Rheum. 1981 Dec;24(12):1459–1468. doi: 10.1002/art.1780241201. [DOI] [PubMed] [Google Scholar]
- Yao Q. Y., Rickinson A. B., Gaston J. S., Epstein M. A. In vitro analysis of the Epstein-Barr virus: host balance in long-term renal allograft recipients. Int J Cancer. 1985 Jan 15;35(1):43–49. doi: 10.1002/ijc.2910350108. [DOI] [PubMed] [Google Scholar]


