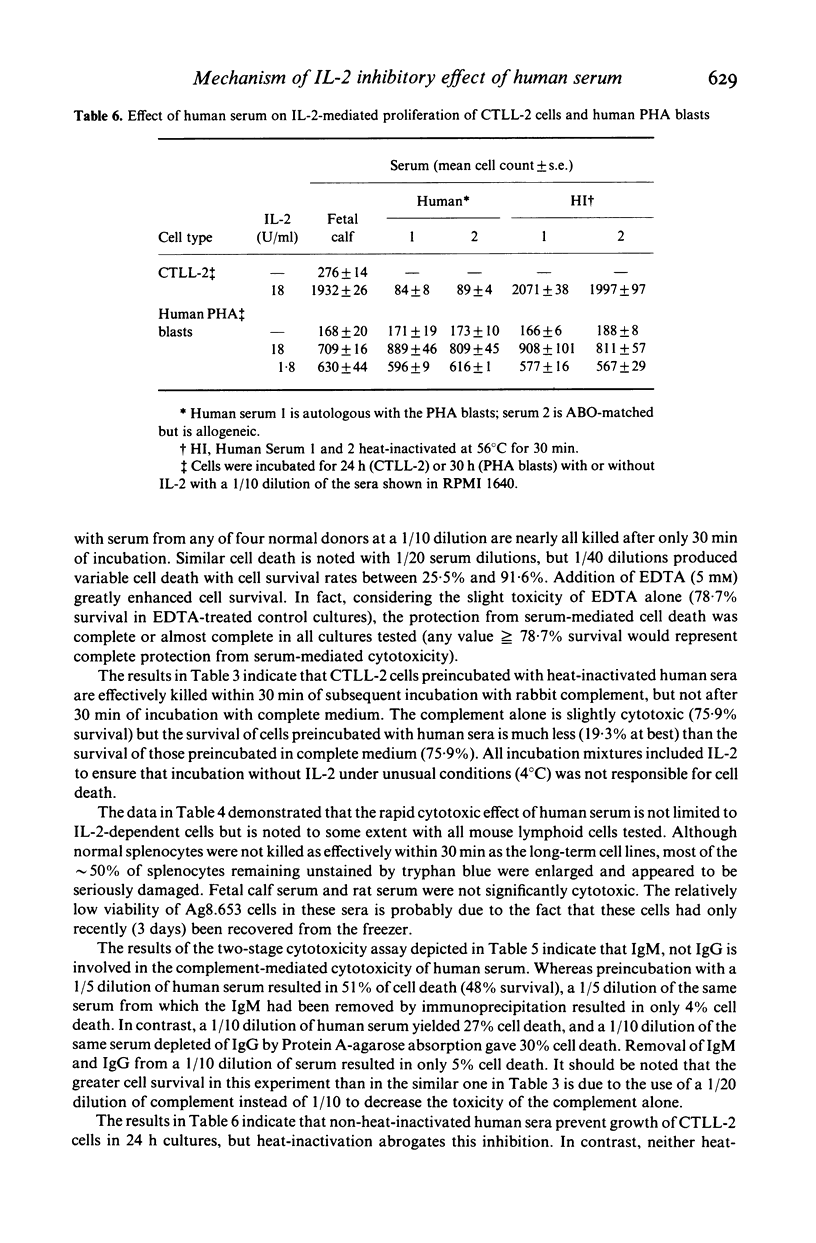
Abstract
A number of investigators have reported that human serum inhibits the proliferation of IL-2-dependent mouse cells in IL-2 bioassays, but the mechanism of inhibition has not been carefully examined. We noticed that IL-2-dependent mouse cells (CTLL-2) are killed within 30 min in the presence of a 1/10 dilution of human serum. However, CTLL-2 cells totally deprived of IL-2 did not begin to die until at least 6 h in culture. Thus, even complete inhibition of IL-2 by human serum could not account for the rapid cytotoxicity caused by human serum. Since humans have 'natural' antibodies which react with mouse cells, it seemed possible that the cytotoxicity was due to antibody/complement-mediated cell lysis. This was supported by the observation that EDTA (at a concentration sufficient to inhibit complement) protected CTLL-2 cells from the cytotoxic effects of human sera from four normal donors. In addition, preincubation of CTLL-2 cells with heat-inactivated human sera at 4 degrees C rendered them much more susceptible to lysis with rabbit complement than cells which were preincubated with complete culture medium. The cytotoxicity of human serum is not limited to IL-2-dependent mouse cells but was also observed with EL4 and Ag8.653 cells as well as normal splenocytes. The cytotoxic effect of human serum was lost upon removal of IgM, but not upon removal of IgG. These results strongly suggest that the inhibition of proliferation of IL-2-dependent mouse cells by human serum is due to antibody/complement-mediated lysis of those cells. In addition, non-heat-inactivated human serum did not inhibit the IL-2-mediated proliferation of human PHA blasts, indicating that there is no inherent inhibitory activity in human serum apart from the cytotoxic effect on xenogeneic cells. Thus the reported IL-2 inhibitory activity of whole human serum is probably not biologically relevant.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Attallah A. M., Johnson R. P. A simple highly sensitive methods for the determination of cell viability using an electronic particle analyzer, Coulter counter. J Immunol Methods. 1981;41(2):155–162. doi: 10.1016/0022-1759(81)90239-8. [DOI] [PubMed] [Google Scholar]
- Chouaib S., Chatenoud L., Klatzmann D., Fradelizi D. The mechanisms of inhibition of human IL 2 production. II. PGE2 induction of suppressor T lymphocytes. J Immunol. 1984 Apr;132(4):1851–1857. [PubMed] [Google Scholar]
- Djeu J. Y., Kasahara T., Balow J. E., Tsokos G. C. Decreased interleukin 2 inhibitor in sera of patients with autoimmune disorders. Clin Exp Immunol. 1986 Aug;65(2):279–285. [PMC free article] [PubMed] [Google Scholar]
- Gullberg M., Smith K. A. Regulation of T cell autocrine growth. T4+ cells become refractory to interleukin 2. J Exp Med. 1986 Feb 1;163(2):270–284. doi: 10.1084/jem.163.2.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardt C., Röllinghoff M., Pfizenmaier K., Mosmann H., Wagner H. Lyt-23+ cyclophosphamide-sensitive T cells regulate the activity of an interleukin 2 inhibitor in vivo. J Exp Med. 1981 Aug 1;154(2):262–274. doi: 10.1084/jem.154.2.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda M., Chan C., Shevach E. M. Characterization and partial purification of a specific interleukin 2 inhibitor. J Immunol. 1985 Sep;135(3):1834–1839. [PubMed] [Google Scholar]
- Hooton J. W., Riendeau D., Paetkau V. Is IL-2 regulated by a serum inhibitor? Cell Immunol. 1985 Oct 15;95(2):311–321. doi: 10.1016/0008-8749(85)90318-1. [DOI] [PubMed] [Google Scholar]
- Inaba K., Granelli-Piperno A., Steinman R. M. Dendritic cells induce T lymphocytes to release B cell-stimulating factors by an interleukin 2-dependent mechanism. J Exp Med. 1983 Dec 1;158(6):2040–2057. doi: 10.1084/jem.158.6.2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh K., Tilden A. B., Balch C. M. Role of interleukin 2 and a serum suppressive factor on the induction of activated killer cells cytotoxic for autologous human melanoma cells. Cancer Res. 1985 Jul;45(7):3173–3178. [PubMed] [Google Scholar]
- Kearney J. F., Radbruch A., Liesegang B., Rajewsky K. A new mouse myeloma cell line that has lost immunoglobulin expression but permits the construction of antibody-secreting hybrid cell lines. J Immunol. 1979 Oct;123(4):1548–1550. [PubMed] [Google Scholar]
- Kirkman R. L., Barrett L. V., Gaulton G. N., Kelley V. E., Ythier A., Strom T. B. Administration of an anti-interleukin 2 receptor monoclonal antibody prolongs cardiac allograft survival in mice. J Exp Med. 1985 Jul 1;162(1):358–362. doi: 10.1084/jem.162.1.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornfeld H., Berman J. S., Beer D. J., Center D. M. Induction of human T lymphocyte motility by interleukin 2. J Immunol. 1985 Jun;134(6):3887–3890. [PubMed] [Google Scholar]
- Lelchuk R., Playfair J. H. Serum IL-2 inhibitor in mice. I. Increase during infection. Immunology. 1985 Sep;56(1):113–118. [PMC free article] [PubMed] [Google Scholar]
- Lotze M. T., Frana L. W., Sharrow S. O., Robb R. J., Rosenberg S. A. In vivo administration of purified human interleukin 2. I. Half-life and immunologic effects of the Jurkat cell line-derived interleukin 2. J Immunol. 1985 Jan;134(1):157–166. [PubMed] [Google Scholar]
- Maddock E. O., Maddock S. W., Kelley V. E., Strom T. B. Rapid stereospecific stimulation of lymphocytic metabolism by interleukin 2. J Immunol. 1985 Dec;135(6):4004–4008. [PubMed] [Google Scholar]
- Miller R. A., Rozans M. K., Ythier A. A., Strom T. B. Stages of T cell activation: continued antigen dependence of IL 2-producing cells after IL 2 receptor expression. J Immunol. 1986 Feb 1;136(3):977–983. [PubMed] [Google Scholar]
- Pruett S. B., Lackey A., Howell B., Ainsworth J. A quantitative, non-isotopic bioassay for interleukin 2. Immunol Invest. 1985 Dec;14(6):541–548. doi: 10.3109/08820138509022682. [DOI] [PubMed] [Google Scholar]
- Pruett S. B., Wolcott M. The heterophile transplantation antigen (HT-A) system: a unique heterophile system exhibiting multiple specificities. Tissue Antigens. 1982 Aug;20(2):112–122. doi: 10.1111/j.1399-0039.1982.tb00334.x. [DOI] [PubMed] [Google Scholar]
- Rosenberg S. A., Grimm E. A., McGrogan M., Doyle M., Kawasaki E., Koths K., Mark D. F. Biological activity of recombinant human interleukin-2 produced in Escherichia coli. Science. 1984 Mar 30;223(4643):1412–1414. doi: 10.1126/science.6367046. [DOI] [PubMed] [Google Scholar]
- Rosenberg S. A., Mulé J. J., Spiess P. J., Reichert C. M., Schwarz S. L. Regression of established pulmonary metastases and subcutaneous tumor mediated by the systemic administration of high-dose recombinant interleukin 2. J Exp Med. 1985 May 1;161(5):1169–1188. doi: 10.1084/jem.161.5.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouse B. T., Miller L. S., Turtinen L., Moore R. N. Augmentation of immunity to herpes simplex virus by in vivo administration of interleukin 2. J Immunol. 1985 Feb;134(2):926–930. [PubMed] [Google Scholar]
- Wang A., Lu S. D., Mark D. F. Site-specific mutagenesis of the human interleukin-2 gene: structure-function analysis of the cysteine residues. Science. 1984 Jun 29;224(4656):1431–1433. doi: 10.1126/science.6427925. [DOI] [PubMed] [Google Scholar]
- Watson J. Continuous proliferation of murine antigen-specific helper T lymphocytes in culture. J Exp Med. 1979 Dec 1;150(6):1510–1519. doi: 10.1084/jem.150.6.1510. [DOI] [PMC free article] [PubMed] [Google Scholar]


