Abstract
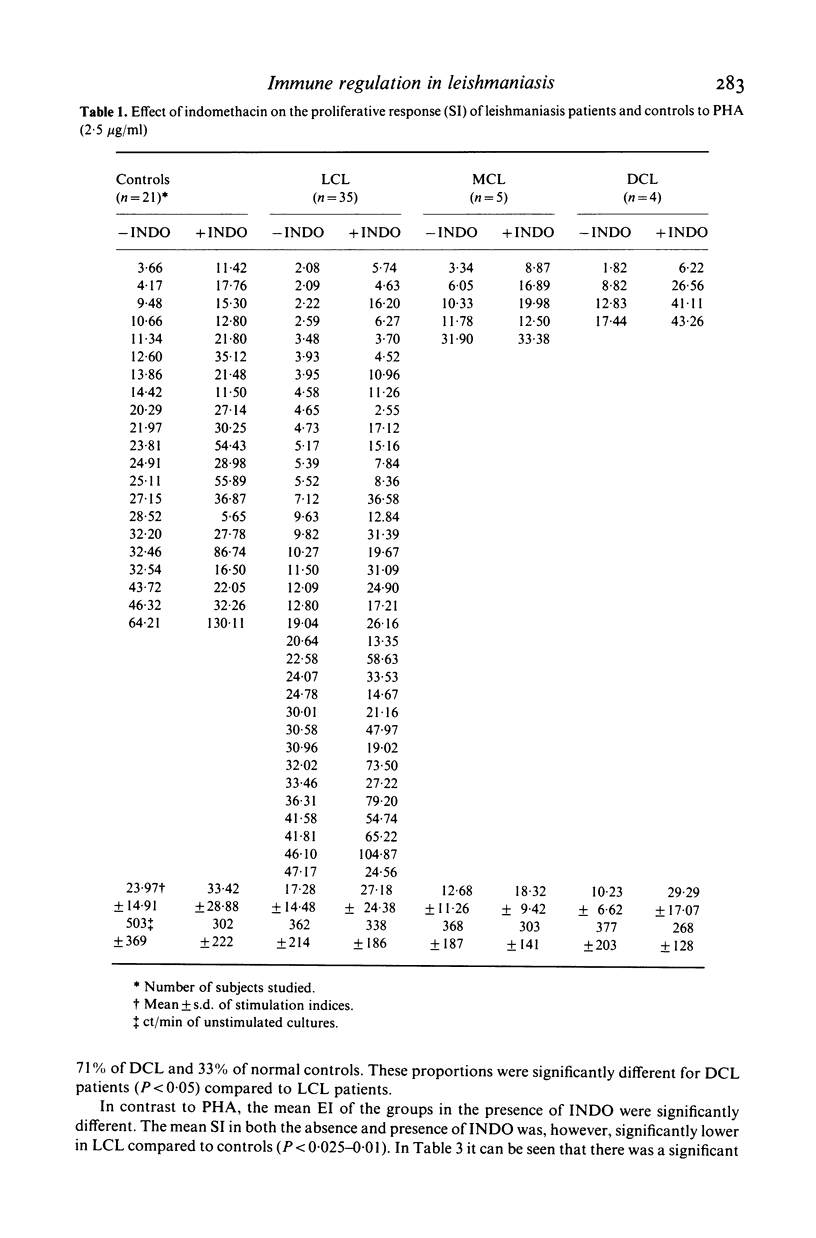
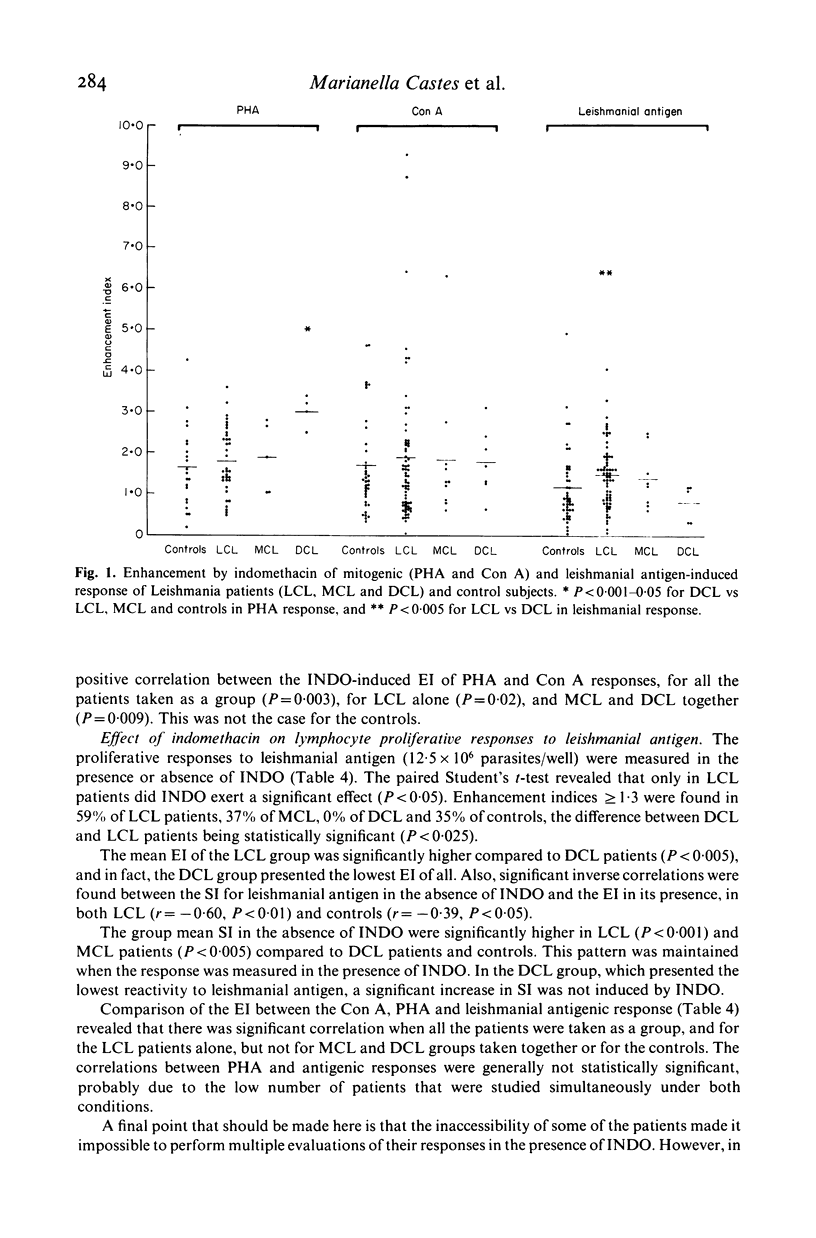
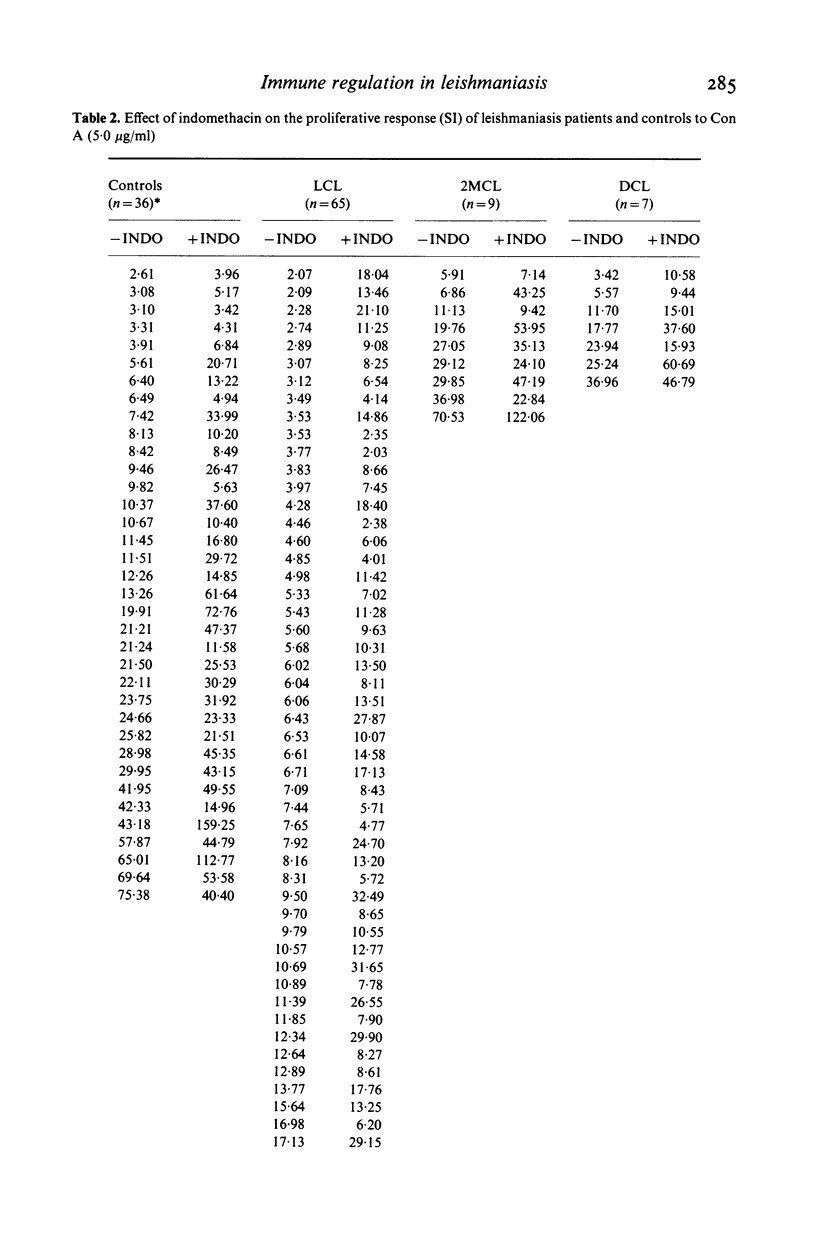
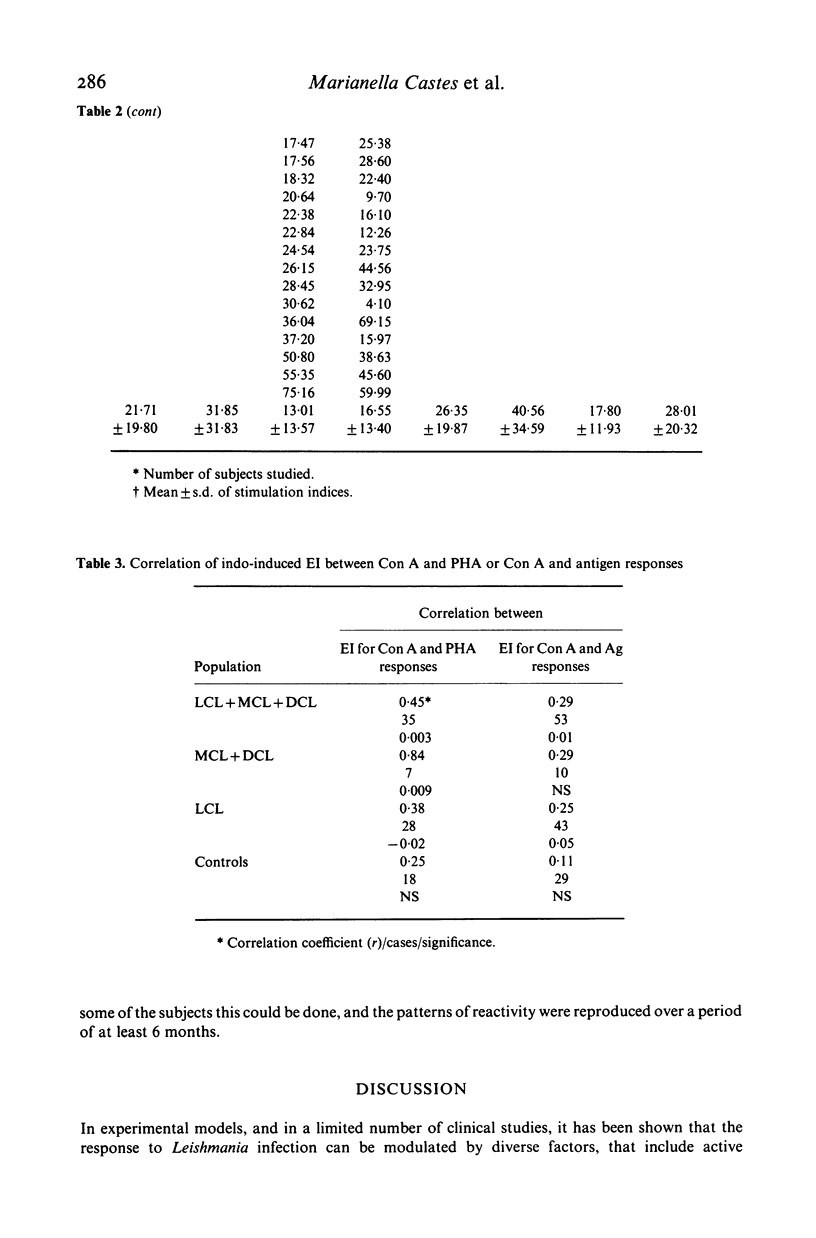
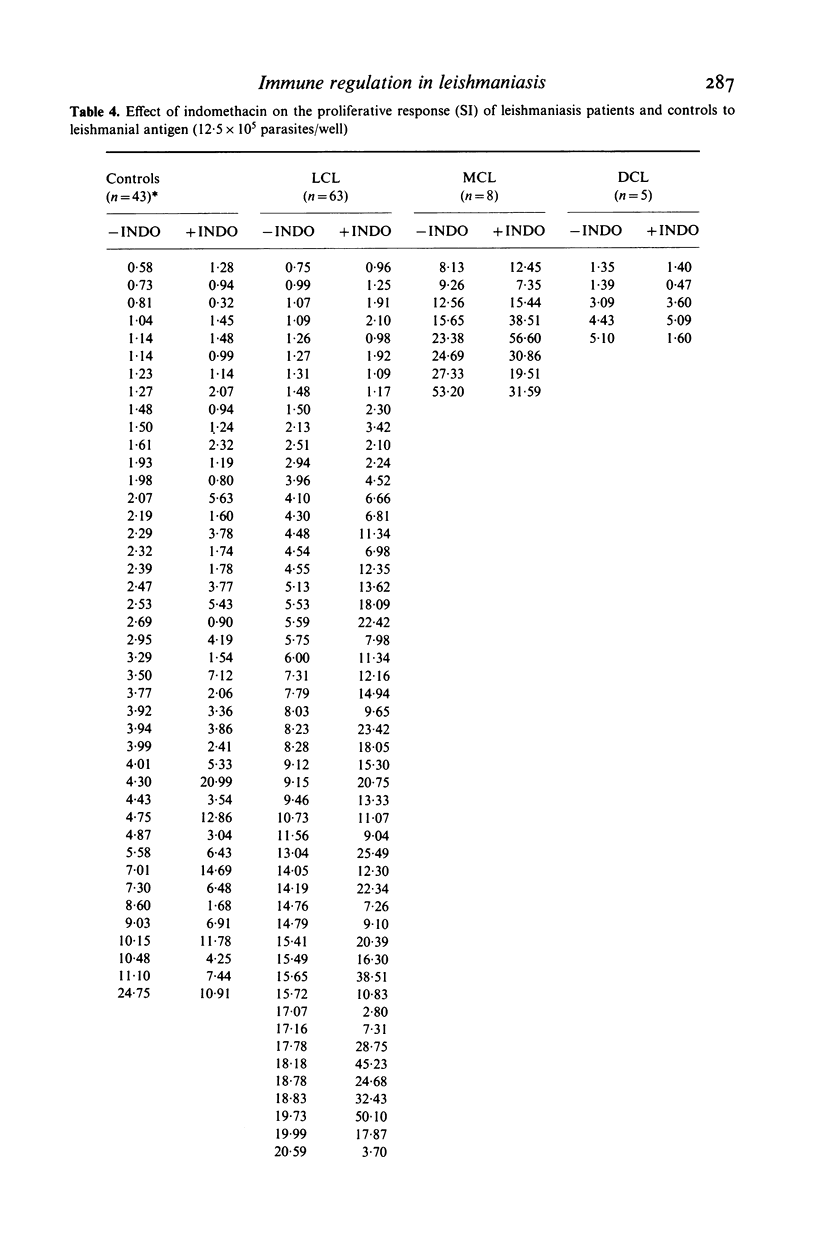
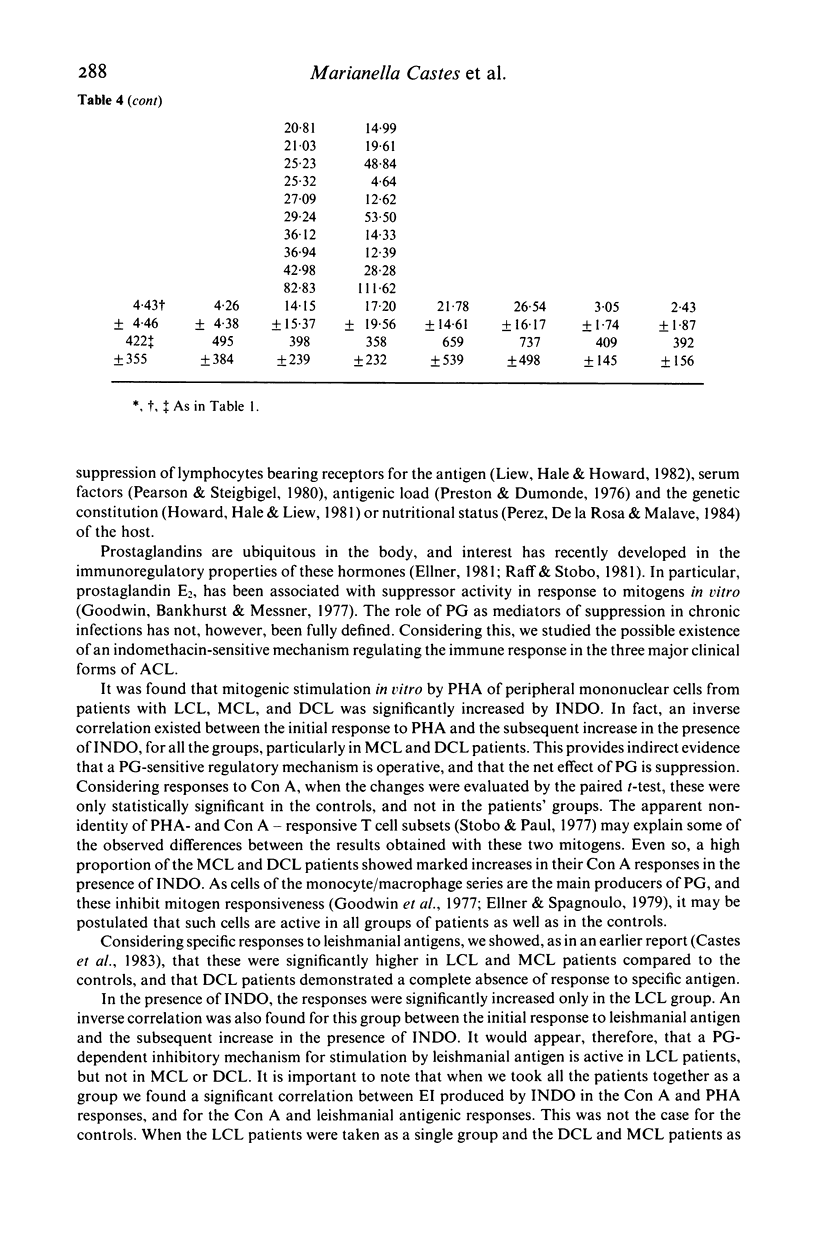
We investigated some aspects of the regulation of the immune response that were sensitive to the effect of indomethacin (INDO), an inhibitor of prostaglandin synthesis, in 84 patients with American cutaneous leishmaniasis (ACL), and in normal controls. The patients were classified on the basis of clinical and histopathological criteria as suffering localized (LCL), mucocutaneous (MCL) or diffuse (DCL) forms of the disease. The responses in vitro to mitogens (PHA and Con A) and leishmanial antigens were evaluated in the presence or absence of INDO. It was found that the drug significantly increased in vitro the mitogenic stimulation by PHA of peripheral blood mononuclear cells from LCL, MCL and DCL patients, but the effect was less evident in the controls. Considering specific responses to leishmanial antigens, we showed that in the presence of INDO, these were significantly increased in LCL patients, but not in MCL or DCL. Also, only in LCL was an inverse correlation found between the initial response to leishmanial antigen and the increase caused by INDO. Significant correlations were found between the INDO-induced enhancement of PHA and Con A responses in the patient groups, but not in the controls. In LCL patients there was a significant correlation between the increases caused by INDO of the mitogen and antigen responses. It can be suggested that an indomethacin-sensitive (prostaglandin dependent) suppressor mechanism operates in LCL patients, that is possibly responsible for the modulation of the immune response against the parasite. In MCL, where this suppressive mechanism is apparently not functional, the response to the parasite is intense, and a possible consequence of this could be tissue damage. Our results indicate, however, that the anergy observed in DCL patients is not due to an involvement of prostaglandins in the suppression of the specific immune response.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bahr G. M., Rook G. A., Stanford J. L. Prostaglandin-dependent regulation of the in vitro proliferative response to mycobacterial antigens of peripheral blood lymphocytes from normal donors and from patients with tuberculosis or leprosy. Clin Exp Immunol. 1981 Sep;45(3):646–653. [PMC free article] [PubMed] [Google Scholar]
- Barsoum I. S., Todd C. W., Habib M., El Alamy M. A., Colley D. G. The effects of indomethacin on in vitro peripheral blood mononuclear cell reactivity in human schistosomiasis. Parasite Immunol. 1983 Sep;5(5):441–447. doi: 10.1111/j.1365-3024.1983.tb00759.x. [DOI] [PubMed] [Google Scholar]
- Carvalho E. M., Johnson W. D., Barreto E., Marsden P. D., Costa J. L., Reed S., Rocha H. Cell mediated immunity in American cutaneous and mucosal leishmaniasis. J Immunol. 1985 Dec;135(6):4144–4148. [PubMed] [Google Scholar]
- Castes M., Agnelli A., Verde O., Rondón A. J. Characterization of the cellular immune response in American cutaneous leishmaniasis. Clin Immunol Immunopathol. 1983 May;27(2):176–186. doi: 10.1016/0090-1229(83)90068-5. [DOI] [PubMed] [Google Scholar]
- Castés M., Agnelli A., Rondón A. J. Mechanisms associated with immunoregulation in human American cutaneous leishmaniasis. Clin Exp Immunol. 1984 Aug;57(2):279–286. [PMC free article] [PubMed] [Google Scholar]
- Damle N. K., Mohagheghpour N., Kansas G. S., Fishwild D. M., Engleman E. G. Immunoregulatory T cell circuits in man. Identification of a distinct T cell subpopulation of the helper/inducer lineage that amplifies the development of alloantigen-specific suppressor T cells. J Immunol. 1985 Jan;134(1):235–243. [PubMed] [Google Scholar]
- Ellner J. J. Suppressor cells of man. Clin Immunol Rev. 1981;1(1):119–214. [PubMed] [Google Scholar]
- Goodwin J. S., Bankhurst A. D., Messner R. P. Suppression of human T-cell mitogenesis by prostaglandin. Existence of a prostaglandin-producing suppressor cell. J Exp Med. 1977 Dec 1;146(6):1719–1734. doi: 10.1084/jem.146.6.1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin J. S., Ceuppens J. Regulation of the immune response by prostaglandins. J Clin Immunol. 1983 Oct;3(4):295–315. doi: 10.1007/BF00915791. [DOI] [PubMed] [Google Scholar]
- Liew F. Y., Hale C., Howard J. G. Immunologic regulation of experimental cutaneous leishmaniasis. V. Characterization of effector and specific suppressor T cells. J Immunol. 1982 Apr;128(4):1917–1922. [PubMed] [Google Scholar]
- Pearson R. D., Steigbigel R. T. Mechanism of lethal effect of human serum upon Leishmania donovani. J Immunol. 1980 Nov;125(5):2195–2201. [PubMed] [Google Scholar]
- Petersen E. A., Neva F. A., Barral A., Correa-Coronas R., Bogaert-Diaz H., Martinez D., Ward F. E. Monocyte suppression of antigen-specific lymphocyte responses in diffuse cutaneous leishmaniasis patients from the Dominican Republic. J Immunol. 1984 May;132(5):2603–2606. [PubMed] [Google Scholar]
- Petersen E. A., Neva F. A., Oster C. N., Bogaert Diaz H. Specific inhibition of lymphocyte-proliferation responses by adherent suppressor cells in diffuse cutaneous leishmaniasis. N Engl J Med. 1982 Feb 18;306(7):387–392. doi: 10.1056/NEJM198202183060702. [DOI] [PubMed] [Google Scholar]
- Pérez H., De La Rosa M., Malavé I. The effect of protein restriction on the development of protective immunity to Leishmania mexicana. Parasite Immunol. 1984 Jul;6(4):285–294. doi: 10.1111/j.1365-3024.1984.tb00801.x. [DOI] [PubMed] [Google Scholar]
- Steiger R. F., Steiger E. Cultivation of Leishmania donovani and Leishmania braziliensis in defined media: nutritional requirements. J Protozool. 1977 Aug;24(3):437–441. doi: 10.1111/j.1550-7408.1977.tb04771.x. [DOI] [PubMed] [Google Scholar]
- Stobo J. D., Paul W. E. Functional heterogeneity of murine lymphoid cells. 3. Differential responsiveness of T cells to phytohemagglutinin and concanavalin A as a probe for T cell subsets. J Immunol. 1973 Feb;110(2):362–375. [PubMed] [Google Scholar]
- Vosixa G., Thies J. Effect of indomethacin on blastogenesis of lymphocytes from cancer patients: differentiation of patient types. Clin Immunol Immunopathol. 1979 May;13(1):30–38. doi: 10.1016/0090-1229(79)90017-5. [DOI] [PubMed] [Google Scholar]


