Abstract
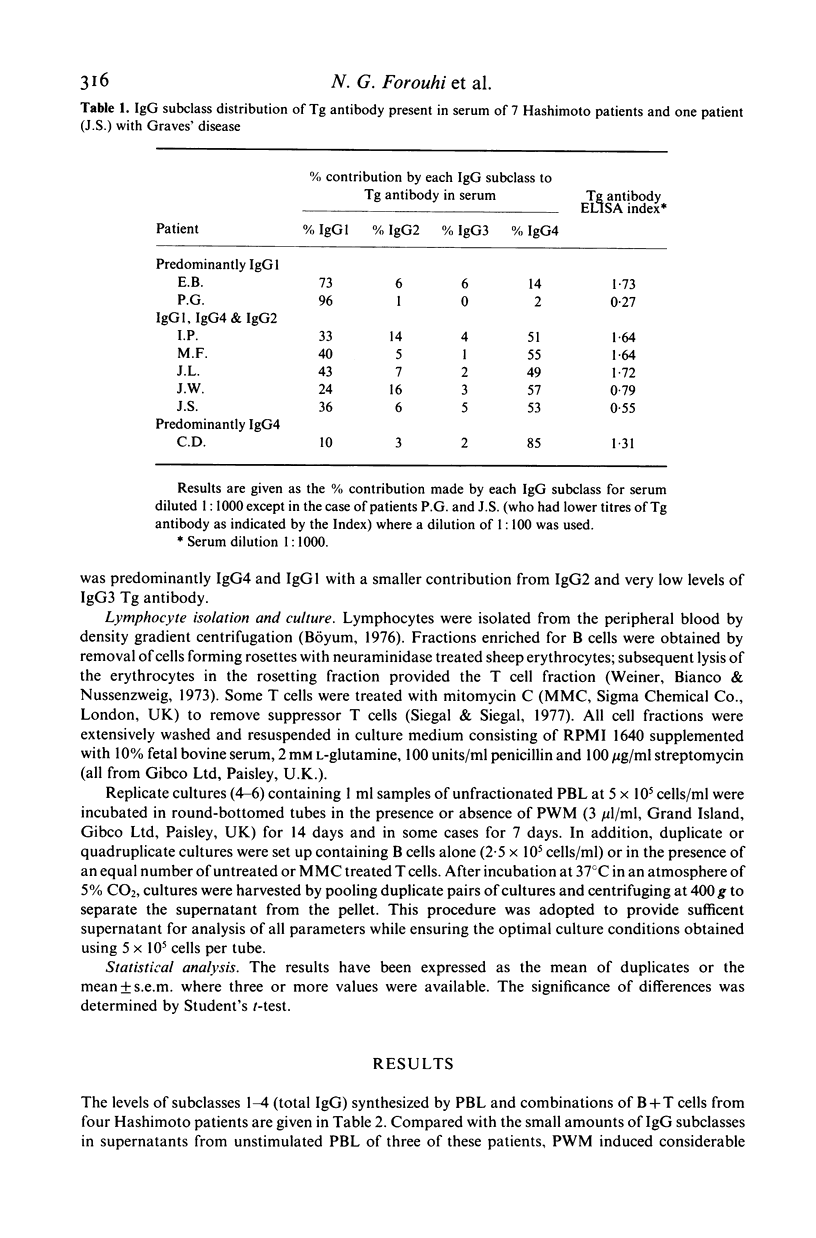
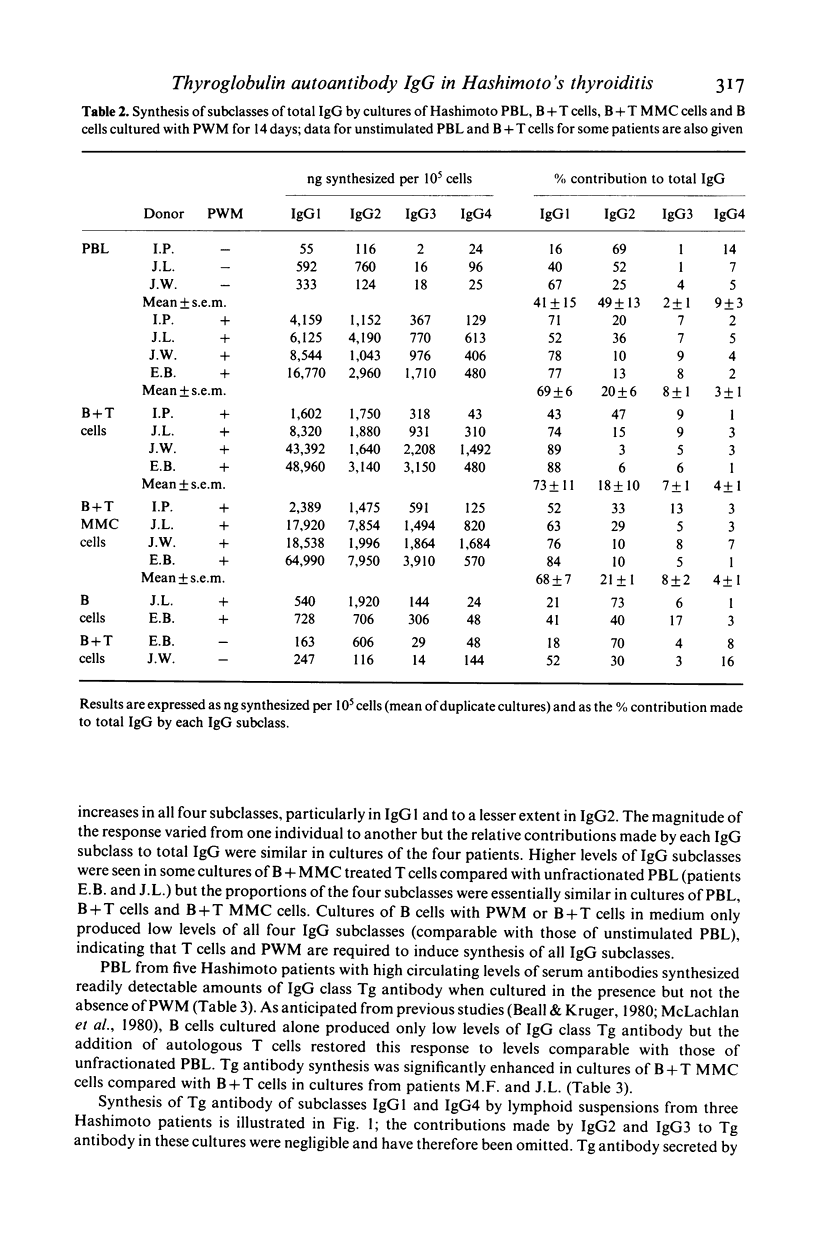
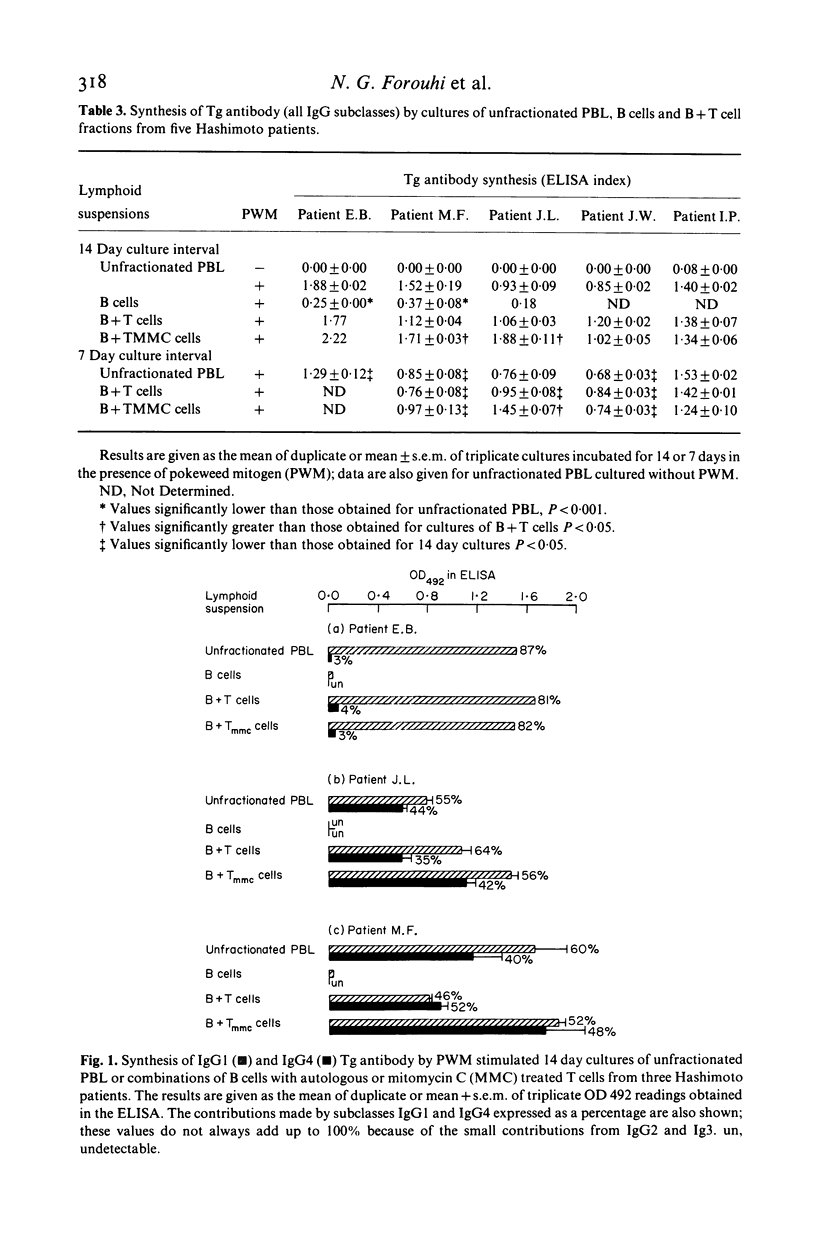
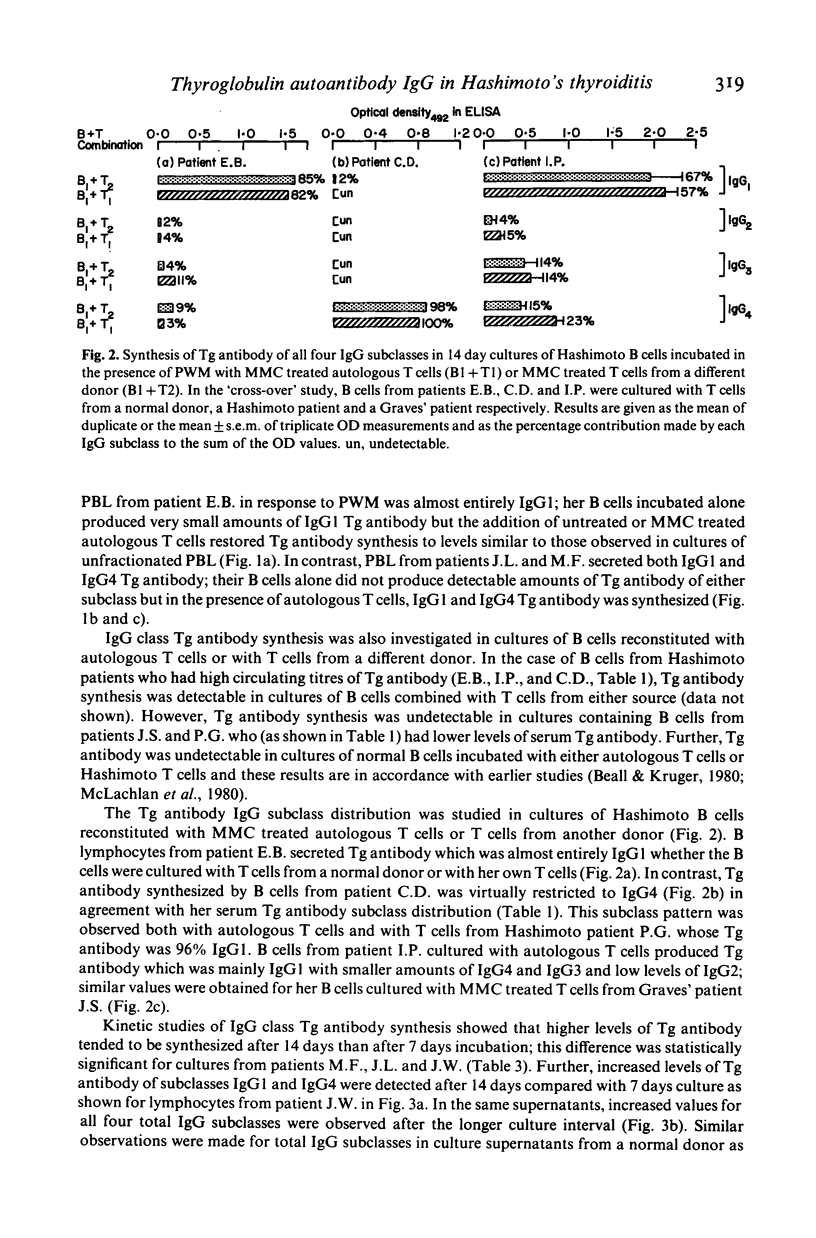
Microsomal and thyroglobulin (Tg) antibodies in patients with autoimmune thyroid disease are usually predominantly of subclasses IgG1 and/or IgG4 and the distribution pattern is characteristic for the serum of an individual. We have studied the role of T cells in synthesis of total IgG and Tg antibody IgG subclasses (measured by ELISA) in cultures of peripheral blood lymphocytes (PBL) from Hashimoto patients. Unfractionated PBL incubated with the T dependent activator pokeweed mitogen (PWM) synthesized IgG of all four IgG subclasses in the proportions 69% IgG1, 20% IgG2, 8% IgG3 and 3% IgG4; these values are similar to the proportions of the subclasses in serum. In contrast, the IgG subclass of Tg antibody was predominantly IgG1 in one patient, approximately equal proportions of IgG1 and IgG4 in four patients, and almost completely restricted to IgG4 in one patient; these patterns were similar to the subclass distribution of the autoantibodies in the individual patients' serum. B cells incubated alone secreted little Tg antibody but the response could be restored to the original levels and proportions of IgG1 and/or IgG4 Tg antibody by the addition of T cells either from the same individual or from another donor. Further, removal of suppressor T cells had little effect on the proportions of IgG1 and IgG4 Tg antibody although the total amounts of Tg antibody of both subclasses were sometimes increased. Our studies indicate that T cells are required in this in vitro system to elicit Tg antibody synthesis and to control the magnitude of the antibody response. However, the characteristic IgG subclass distribution of Tg antibody in an individual is determined at the level of the B cell.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atherton M. C., McLachlan S. M., Pegg C. A., Dickinson A., Baylis P., Young E. T., Proctor S. J., Rees Smith B. Thyroid autoantibody synthesis by lymphocytes from different lymphoid organs: fractionation of B cells on density gradients. Immunology. 1985 Jun;55(2):271–279. [PMC free article] [PubMed] [Google Scholar]
- Barrett D. J., Ayoub E. M. IgG2 subclass restriction of antibody to pneumococcal polysaccharides. Clin Exp Immunol. 1986 Jan;63(1):127–134. [PMC free article] [PubMed] [Google Scholar]
- Beall G. N., Kruger S. R. Production of human antithyroglobulin in vitro. II. Regulation by T cells. Clin Immunol Immunopathol. 1980 Aug;16(4):498–503. doi: 10.1016/0090-1229(80)90191-9. [DOI] [PubMed] [Google Scholar]
- Davies T. F., Weber C. M., Wallack P., Platzer M. Restricted heterogeneity and T cell dependence of human thyroid autoantibody immunoglobulin G subclasses. J Clin Endocrinol Metab. 1986 May;62(5):945–949. doi: 10.1210/jcem-62-5-945. [DOI] [PubMed] [Google Scholar]
- Endoh M., Sakai H., Nomoto Y., Tomino Y., Kaneshige H. IgA-specific helper activity of T alpha cells in human peripheral blood. J Immunol. 1981 Dec;127(6):2612–2613. [PubMed] [Google Scholar]
- Farrant J., Bryant A. E., Chan J., Himsworth R. L. Thyroglobulin-treated blood dendritic cells induce IgG anti-thyroglobulin antibody in vitro in Hashimoto's thyroiditis. Clin Immunol Immunopathol. 1986 Dec;41(3):433–442. doi: 10.1016/0090-1229(86)90014-0. [DOI] [PubMed] [Google Scholar]
- Ferrante A., Rowan-Kelly B., Beard L. J., Maxwell G. M. An enzyme-linked immunosorbent assay for the quantitation of human IgG subclasses using monoclonal antibodies. J Immunol Methods. 1986 Nov 6;93(2):207–212. doi: 10.1016/0022-1759(86)90190-0. [DOI] [PubMed] [Google Scholar]
- Isakson P. C., Puré E., Vitetta E. S., Krammer P. H. T cell-derived B cell differentiation factor(s). Effect on the isotype switch of murine B cells. J Exp Med. 1982 Mar 1;155(3):734–748. doi: 10.1084/jem.155.3.734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krömer G., Sundick R. S., Schauenstein K., Hála K., Wick G. Analysis of lymphocytes infiltrating the thyroid gland of Obese strain chickens. J Immunol. 1985 Oct;135(4):2452–2457. [PubMed] [Google Scholar]
- Mayumi M., Kuritani T., Kubagawa H., Cooper M. D. IgG subclass expression by human B lymphocytes and plasma cells: B lymphocytes precommitted to IgG subclass can be preferentially induced by polyclonal mitogens with T cell help. J Immunol. 1983 Feb;130(2):671–677. [PubMed] [Google Scholar]
- McLachlan S. M., Fawcett J., Atherton M. C., Thompson P., Baylis P., Smith B. R. Thyroid autoantibody synthesis by cultures of thyroid and peripheral blood lymphocytes. II. Effect of thyroglobulin on thyroglobulin antibody synthesis. Clin Exp Immunol. 1983 Jun;52(3):620–628. [PMC free article] [PubMed] [Google Scholar]
- McLachlan S. M., Feldt-Rasmussen U., Young E. T., Middleton S. L., Dlichert-Toft M., Siersboek-Nielsen K., Date J., Carr D., Clark F., Rees Smith B. IgG subclass distribution of thyroid autoantibodies: a 'fingerprint' of an individual's response to thyroglobulin and thyroid microsomal antigen. Clin Endocrinol (Oxf) 1987 Mar;26(3):335–346. doi: 10.1111/j.1365-2265.1987.tb00791.x. [DOI] [PubMed] [Google Scholar]
- McLachlan S. M., Proud G., Pegg C. A., Clark F., Rees Smith B. Functional analysis of T and B cells from blood and thyroid tissue in Hashimoto's disease. Clin Exp Immunol. 1985 Mar;59(3):585–592. [PMC free article] [PubMed] [Google Scholar]
- Parkes A. B., McLachlan S. M., Bird P., Rees Smith B. The distribution of microsomal and thyroglobulin antibody activity among the IgG subclasses. Clin Exp Immunol. 1984 Jul;57(1):239–243. [PMC free article] [PubMed] [Google Scholar]
- Siegal F. P., Siegal M. Enhancement by irradiated T cells of human plasma cell production: dissection of helper and suppressor functions in vitro. J Immunol. 1977 Feb;118(2):642–647. [PubMed] [Google Scholar]
- Thompson P. M., McLachlan S. M., Parkes A., Clark F., Howel D., Rees Smith B. The IgG subclass distribution of thyroglobulin antibody synthesized in culture. Scand J Immunol. 1983 Aug;18(2):123–129. doi: 10.1111/j.1365-3083.1983.tb00848.x. [DOI] [PubMed] [Google Scholar]
- Weiss I., De Bernardo E., Davies T. F. Plaque-forming cells in autoimmune thyroid disease. Clin Immunol Immunopathol. 1982 Apr;23(1):50–57. doi: 10.1016/0090-1229(82)90069-1. [DOI] [PubMed] [Google Scholar]


