Abstract
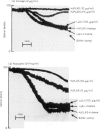
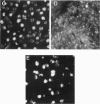
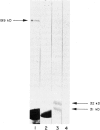
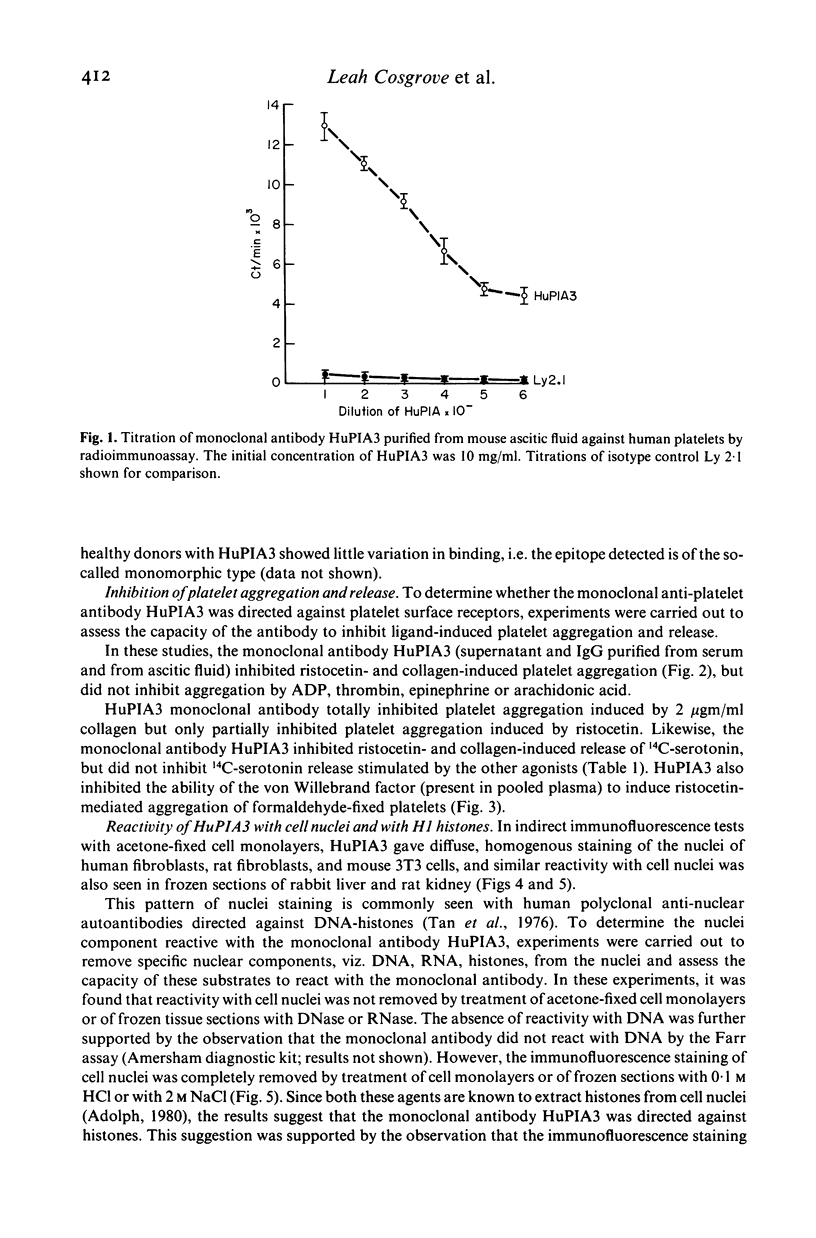
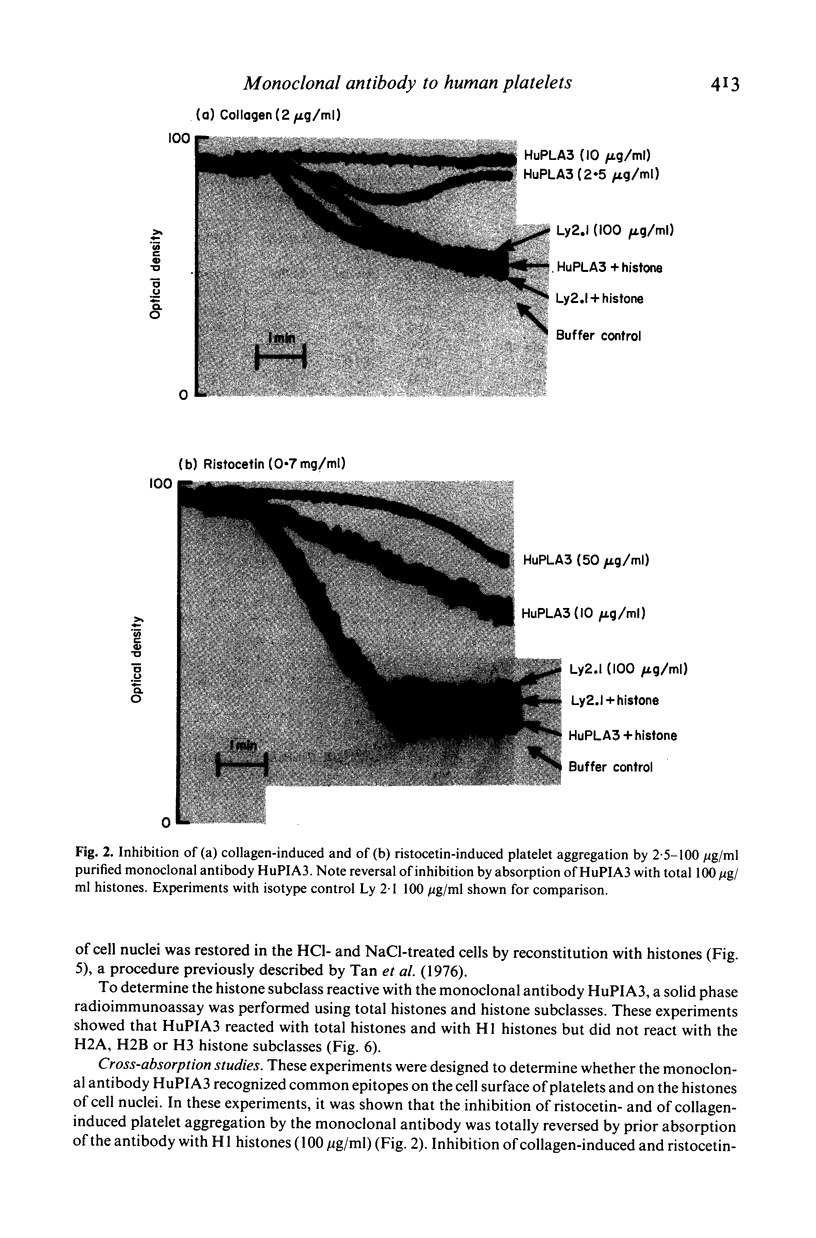
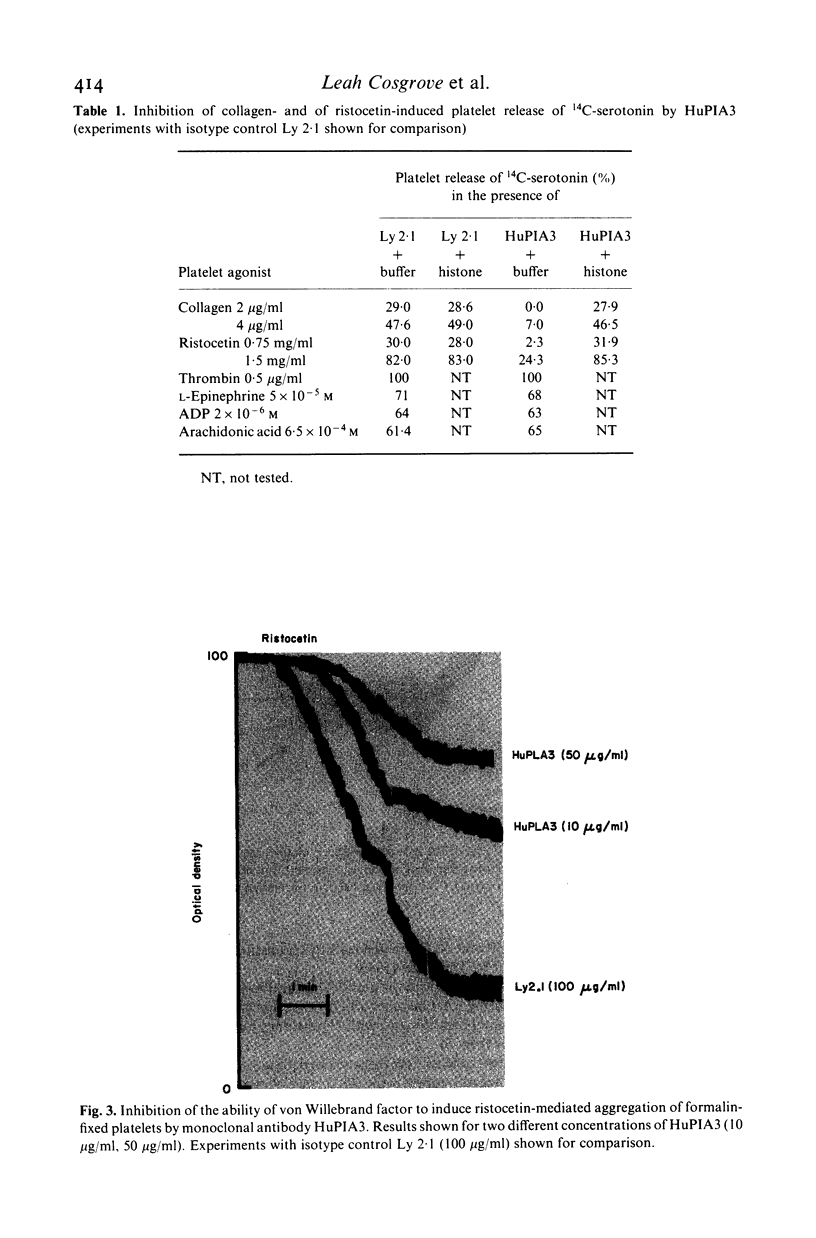
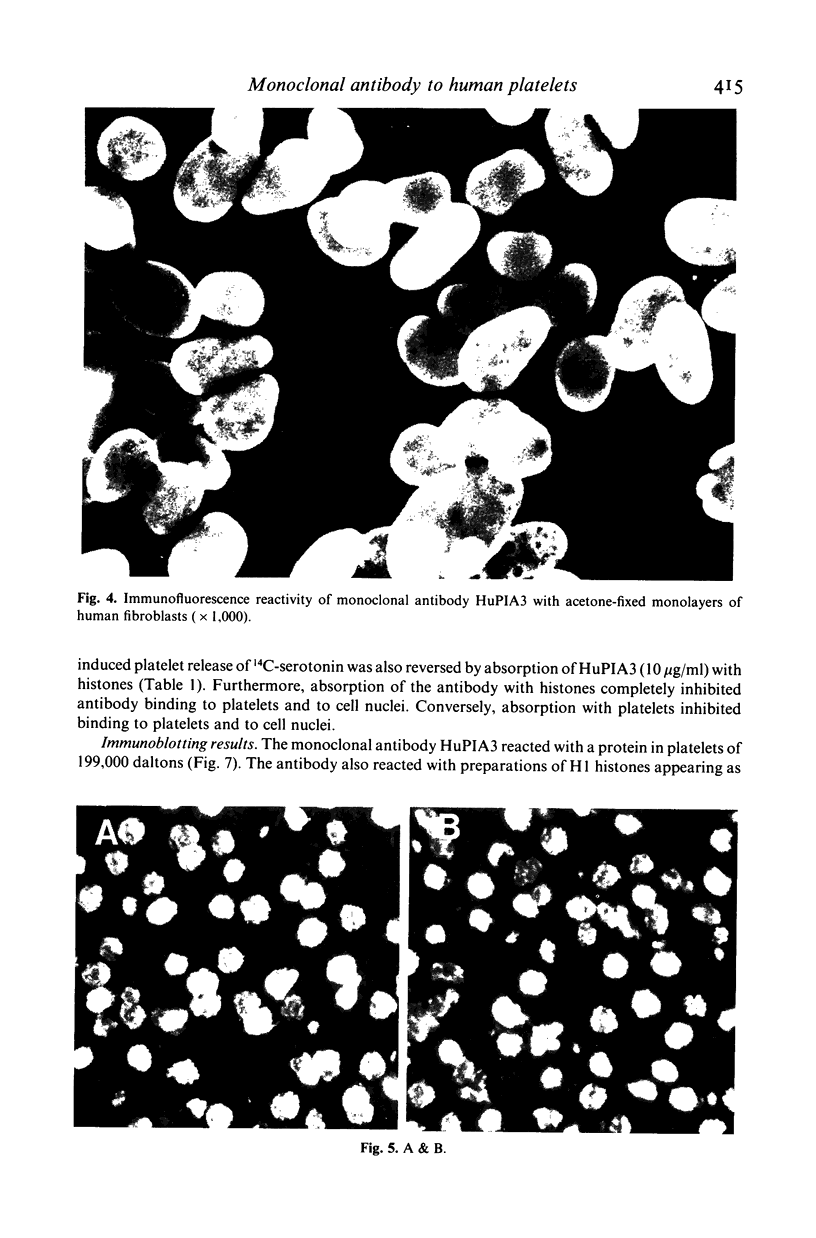
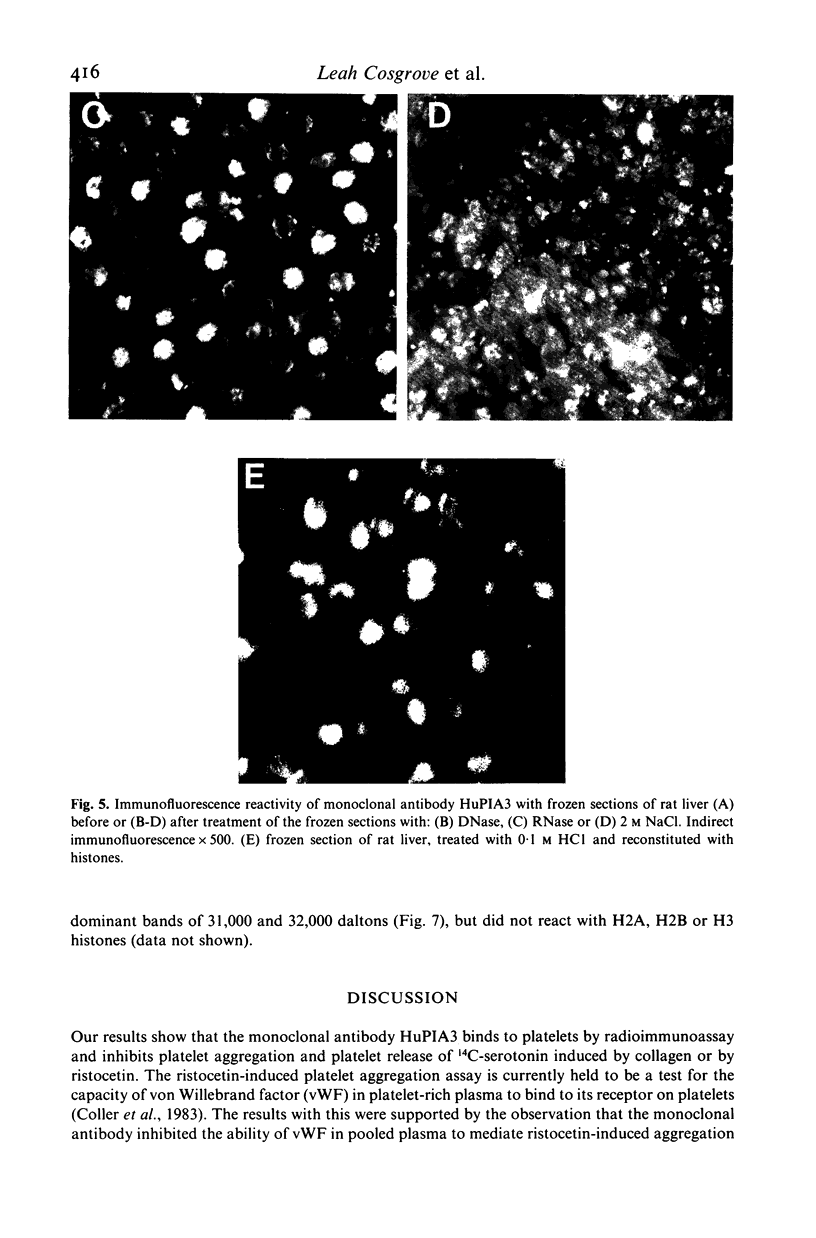
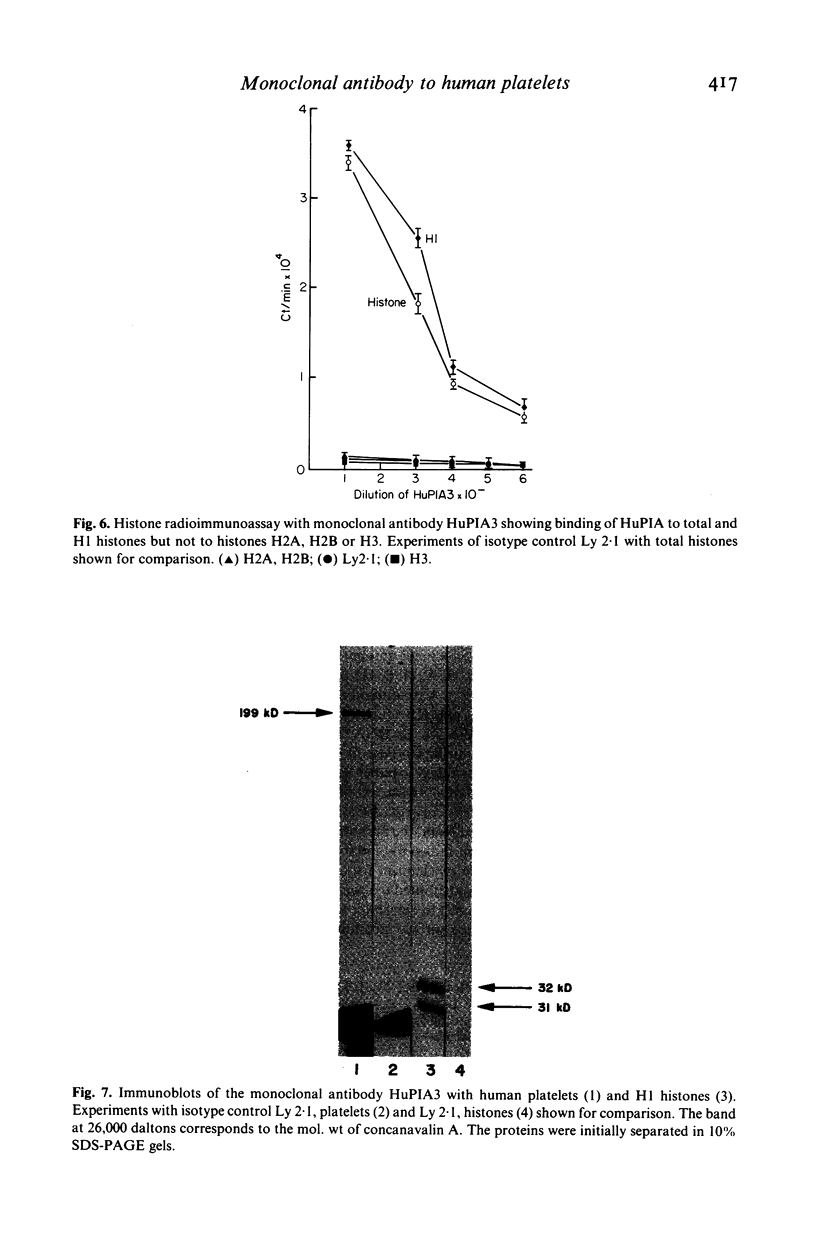
A murine monoclonal antibody HuPIA3, produced by immunization with human platelet membranes, reacted by radioimmunoassay with platelets, and inhibited ristocetin- and collagen-induced platelet aggregation and release of 14C-serotonin. The antibody also inhibited ristocetin-induced aggregation of washed, formaldehyde-fixed platelets by von Willebrand factor. On cultures of human and rodent fibroblasts, and on frozen sections of rabbit liver and rat kidney, the antibody gave a diffuse, homogenous immunofluorescence staining of cell nuclei which could be abolished by treatment with 0.1 M HC1 or 2 M NaCl and restored by reconstitution with histones, suggesting a reaction with nuclear histones. Absorption of the antibody with histones abolished nuclear staining and abrogated the inhibitory effect of the antibody on ristocetin- and collagen-induced platelet aggregation and 14C-serotonin release. Conversely, absorption with platelets removed antibody reactivity for platelets and for cell nuclei. In addition, the antibody reacted with H1 histones by radioimmunoassay, and immunoblotting studies showed that the antibody reacted with a protein of 199,000 daltons on platelets and with H1 histones (31,000 dalton and 32,000 dalton). These observations suggest that the antibody recognizes epitopes found on surface molecules of platelets as well as on H1 histones of cell nuclei.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adolph K. W. Organization of chromosomes in HeLa cells: isolation of histone-depleted nuclei and nuclear scaffolds. J Cell Sci. 1980 Apr;42:291–304. doi: 10.1242/jcs.42.1.291. [DOI] [PubMed] [Google Scholar]
- Agnello V., Arbetter A., Ibanez de Kasep G., Powell R., Tan E. M., Joslin F. Evidence for a subset of rheumatoid factors that cross-react with DNA-histone and have a distinct cross-idiotype. J Exp Med. 1980 Jun 1;151(6):1514–1527. doi: 10.1084/jem.151.6.1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- André-Schwartz J., Datta S. K., Shoenfeld Y., Isenberg D. A., Stollar B. D., Schwartz R. S. Binding of cytoskeletal proteins by monoclonal anti-DNA lupus autoantibodies. Clin Immunol Immunopathol. 1984 May;31(2):261–271. doi: 10.1016/0090-1229(84)90246-0. [DOI] [PubMed] [Google Scholar]
- Asano T., Furie B. C., Furie B. Platelet binding properties of monoclonal lupus autoantibodies produced by human hybridomas. Blood. 1985 Dec;66(6):1254–1260. [PubMed] [Google Scholar]
- Bernstein R. M., Hobbs R. N., Lea D. J., Ward D. J., Hughes G. R. Patterns of antihistone antibody specificity in systemic rheumatic disease. I Systemic lupus erythematosus, mixed connective tissue disease, primary sicca syndrome, and rheumatoid arthritis with vasculitis. Arthritis Rheum. 1985 Mar;28(3):285–293. doi: 10.1002/art.1780280308. [DOI] [PubMed] [Google Scholar]
- Chiang T. M., Kang A. H., Dale J. B., Beachey E. H. Immunochemical studies of the purified human platelet receptor for the alpha 1(I)-chain of chick skin collagen. J Immunol. 1984 Aug;133(2):872–876. [PubMed] [Google Scholar]
- Coller B. S., Peerschke E. I., Scudder L. E., Sullivan C. A. Studies with a murine monoclonal antibody that abolishes ristocetin-induced binding of von Willebrand factor to platelets: additional evidence in support of GPIb as a platelet receptor for von Willebrand factor. Blood. 1983 Jan;61(1):99–110. [PubMed] [Google Scholar]
- Hardin J. A., Thomas J. O. Antibodies to histones in systemic lupus erythematosus: localization of prominent autoantigens on histones H1 and H2B. Proc Natl Acad Sci U S A. 1983 Dec;80(24):7410–7414. doi: 10.1073/pnas.80.24.7410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogarth P. M., Edwards J., McKenzie I. F., Goding J. W., Liew F. Y. Monoclonal antibodies to the murine Ly-2.1 cell surface antigen. Immunology. 1982 May;46(1):135–144. [PMC free article] [PubMed] [Google Scholar]
- Holers V. M., Kotzin B. L. Human peripheral blood monocytes display surface antigens recognized by monoclonal antinuclear antibodies. J Clin Invest. 1985 Sep;76(3):991–998. doi: 10.1172/JCI112100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob L., Lety M. A., Louvard D., Bach J. F. Binding of a monoclonal anti-DNA autoantibody to identical protein(s) present at the surface of several human cell types involved in lupus pathogenesis. J Clin Invest. 1985 Jan;75(1):315–317. doi: 10.1172/JCI111692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao K. J., Pizzo S. V., McKee P. A. Demonstration and characterization of specific binding sites for factor VIII/von Willebrand factor on human platelets. J Clin Invest. 1979 Apr;63(4):656–664. doi: 10.1172/JCI109348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan K. L., Nachman R. L. The interaction of platelets and concanavalin A. Thromb Res. 1975 Dec;7(6):847–859. doi: 10.1016/0049-3848(75)90088-2. [DOI] [PubMed] [Google Scholar]
- Kenneally D., Lolait S. J., Toh B. H. Fibroblast monolayers as substrates for the demonstration and analysis of anti-nuclear autoantibodies. J Clin Lab Immunol. 1983 Jan;10(1):47–51. [PubMed] [Google Scholar]
- Krippner H., Springer B., Merle S., Pirlet K. Antibodies to histones of the IgG and IgM class in systemic lupus erythematosus. Clin Exp Immunol. 1984 Oct;58(1):49–56. [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- McEver R. P., Baenziger N. L., Majerus P. W. Isolation and quantitation of the platelet membrane glycoprotein deficient in thrombasthenia using a monoclonal hybridoma antibody. J Clin Invest. 1980 Dec;66(6):1311–1318. doi: 10.1172/JCI109983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rekvig O. P., Hannestad K. Human autoantibodies that react with both cell nuclei and plasma membranes display specificity for the octamer of histones H2A, H2B, H3, and H4 in high salt. J Exp Med. 1980 Dec 1;152(6):1720–1733. doi: 10.1084/jem.152.6.1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin R. L., Balderas R. S., Tan E. M., Dixon F. J., Theofilopoulos A. N. Multiple autoantigen binding capabilities of mouse monoclonal antibodies selected for rheumatoid factor activity. J Exp Med. 1984 May 1;159(5):1429–1440. doi: 10.1084/jem.159.5.1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz R. S., Stollar B. D. Origins of anti-DNA autoantibodies. J Clin Invest. 1985 Feb;75(2):321–327. doi: 10.1172/JCI111704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan E. M., Robinson J., Robitaille P. Studies on antibodies to histones by immunofluorescence. Scand J Immunol. 1976;5(6-7):811–818. doi: 10.1111/j.1365-3083.1976.tb03030.x. [DOI] [PubMed] [Google Scholar]
- Thurlow P. J., Barlow B., Connellan J. M., McKenzie I. F. Detection of glycoprotein IIb and IIIa by monoclonal antibodies. Br J Haematol. 1983 Sep;55(1):123–134. doi: 10.1111/j.1365-2141.1983.tb01230.x. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Underwood J. R., Pedersen J. S., Chalmers P. J., Toh B. H. Hybrids from normal, germ free, nude and neonatal mice produce monoclonal autoantibodies to eight different intracellular structures. Clin Exp Immunol. 1985 May;60(2):417–426. [PMC free article] [PubMed] [Google Scholar]
- Warley A., Cook G. M. The isolation and characterization of plasma membranes from normal and leukaemic cells of mice. Biochim Biophys Acta. 1973 Sep 27;323(1):55–68. doi: 10.1016/0005-2736(73)90431-8. [DOI] [PubMed] [Google Scholar]