Abstract
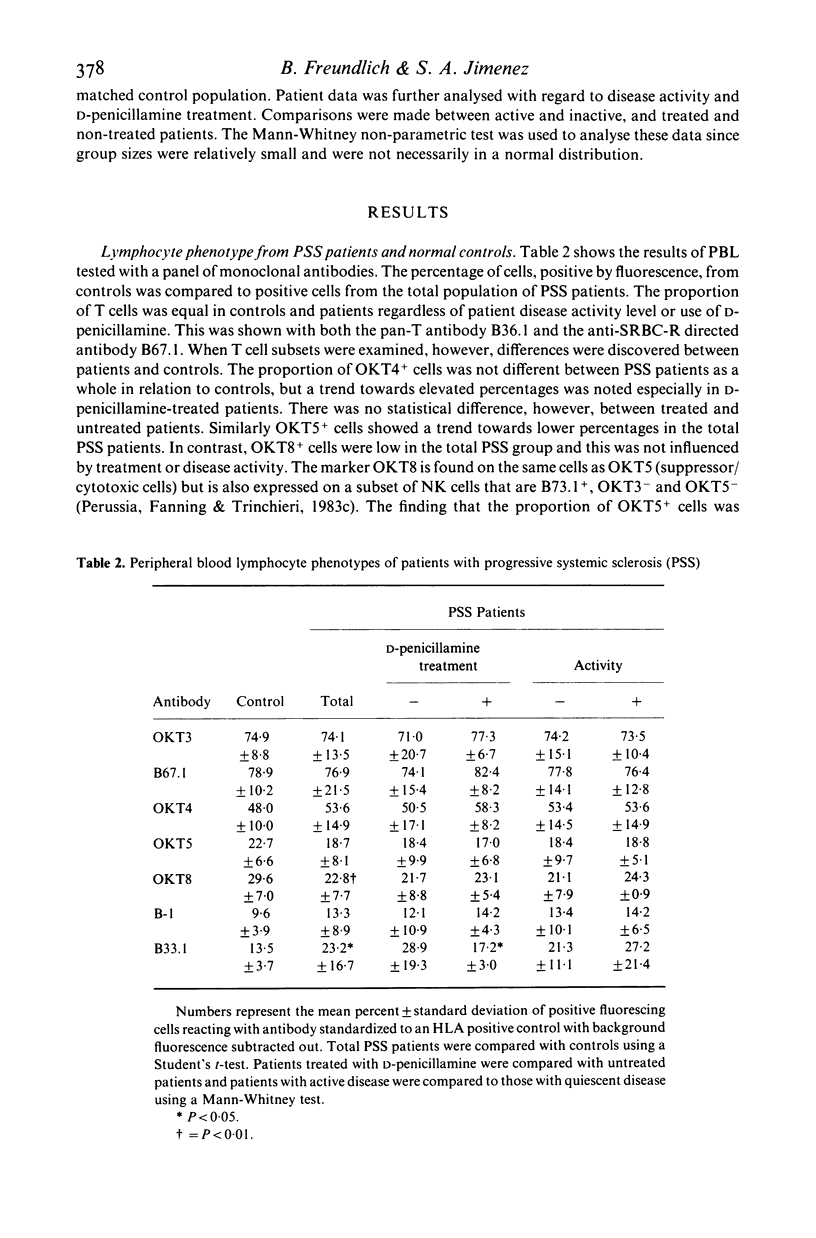
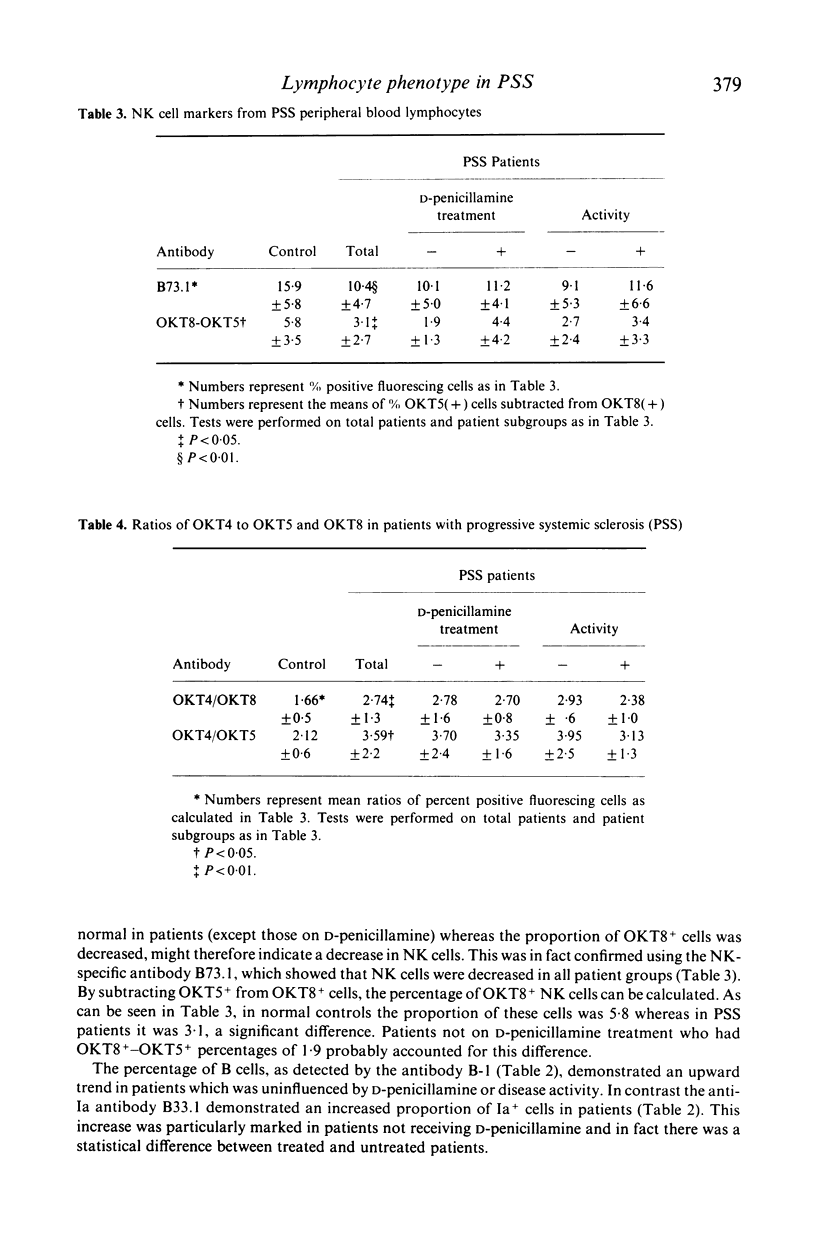
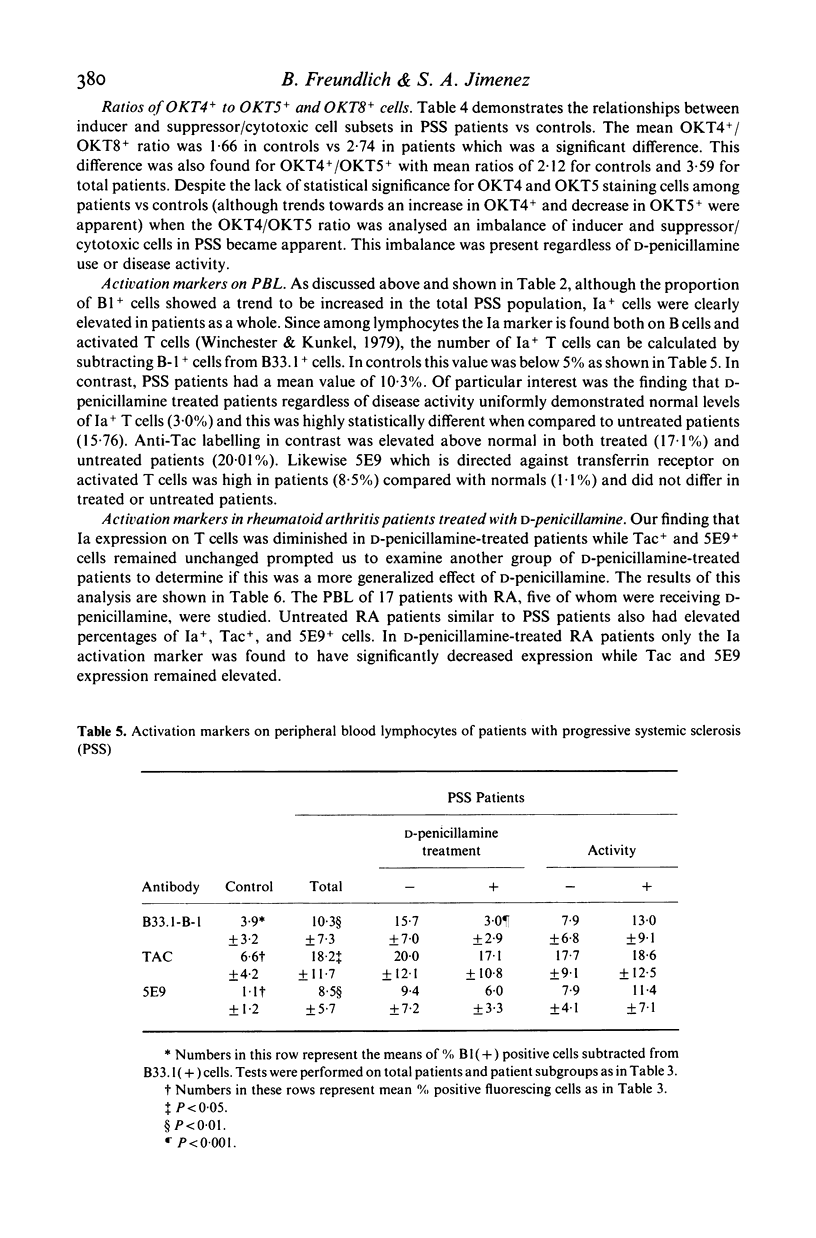
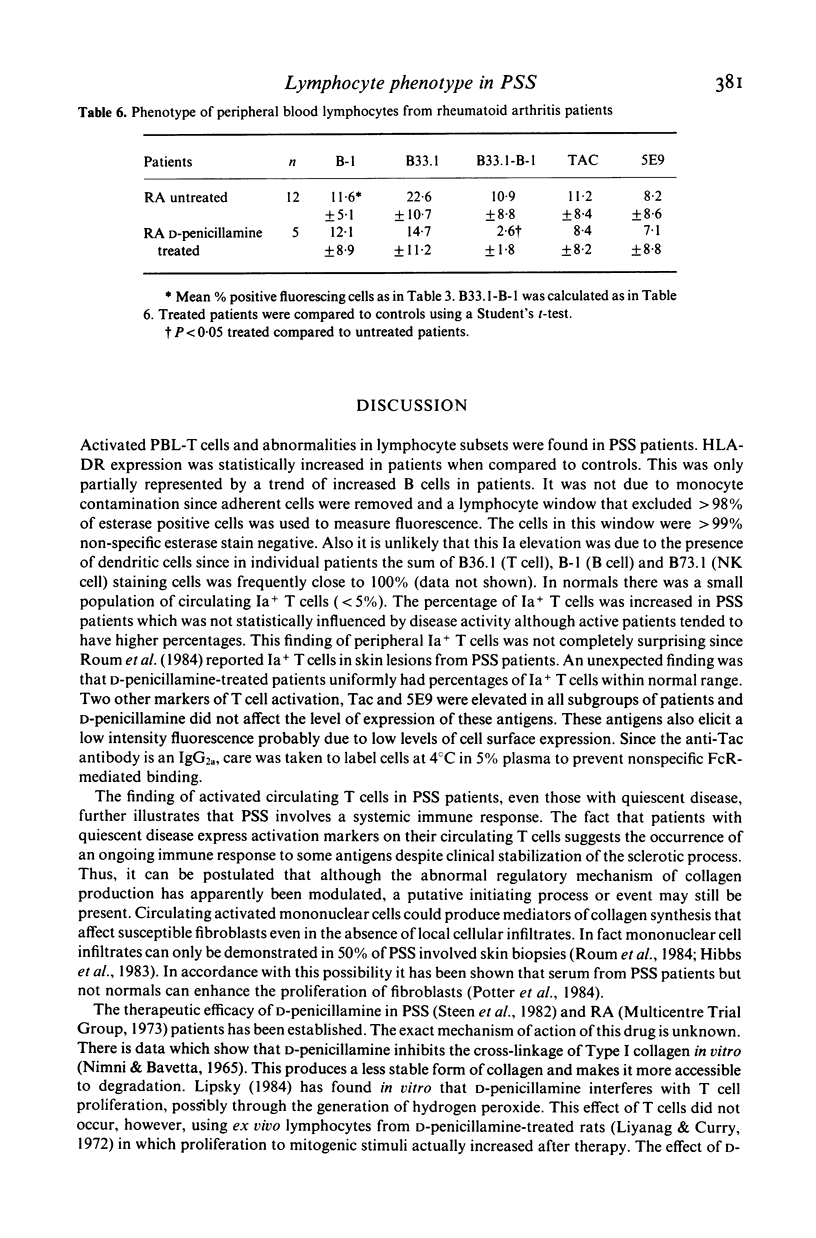
To understand better the role that immune mechanisms could play in the pathogenesis of progressive systemic sclerosis (PSS), the phenotypes of peripheral blood lymphocytes (PBL) from 24 PSS patients and normal controls were compared by a panel of monoclonal antibodies. PBL T cells of patients expressed an increased percentage of several activation markers as defined by monoclonal antibodies B33.1 (anti-Ia), TAC (anti-interleukin 2 receptor) and 5E9 (anti-transferrin receptor). These antigens were also found on PBL of patients with quiescent disease suggesting the persistence of an ongoing immune response despite the absence of clinically apparent disease activity. Patients treated with D-penicillamine had uniformly normal proportions of Ia+ T cells (less than 5%) although the percentage of cells positive for Tac and transferrin receptor continued to be elevated. A second finding was that the percentage of natural killer (NK) cells studied with the monoclonal antibody against NK cells B73.1 (Leu 11c) and particularly an OKT8+ NK cell subset was low in patients (10.4 +/- 4.7) compared with controls (15.9 +/- 5.8). Finally, both treated and untreated patients displayed increased OKT4+/OKT5+ ratios compared to controls. These data suggest that PBL from PSS patients are activated and have abnormal proportions of cell subpopulations. These abnormalities are also present in patients with quiescent disease. The observed effect of D-penicillamine on Ia expression in PSS PBL may reflect a mechanism of action of this drug in the control of autoimmune diseases.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bernstein R. M., Steigerwald J. C., Tan E. M. Association of antinuclear and antinucleolar antibodies in progressive systemic sclerosis. Clin Exp Immunol. 1982 Apr;48(1):43–51. [PMC free article] [PubMed] [Google Scholar]
- Burmester G. R., Yu D. T., Irani A. M., Kunkel H. G., Winchester R. J. Ia+ T cells in synovial fluid and tissues of patients with rheumatoid arthritis. Arthritis Rheum. 1981 Nov;24(11):1370–1376. doi: 10.1002/art.1780241106. [DOI] [PubMed] [Google Scholar]
- D'Angelo W. A., Fries J. F., Masi A. T., Shulman L. E. Pathologic observations in systemic sclerosis (scleroderma). A study of fifty-eight autopsy cases and fifty-eight matched controls. Am J Med. 1969 Mar;46(3):428–440. doi: 10.1016/0002-9343(69)90044-8. [DOI] [PubMed] [Google Scholar]
- FISHER E. R., RODNAN G. P. Pathologic observations concerning the cutaneous lesion of progressive systemic sclerosis: an electron microscopic histochemical and immunohistochemical study. Arthritis Rheum. 1960 Dec;3:536–545. doi: 10.1002/art.1780030607. [DOI] [PubMed] [Google Scholar]
- Fleischmajer R., Perlish J. S., Reeves J. R. Cellular infiltrates in scleroderma skin. Arthritis Rheum. 1977 May;20(4):975–984. doi: 10.1002/art.1780200410. [DOI] [PubMed] [Google Scholar]
- Freundlich B., Trinchieri G., Perussia B., Zurier R. B. The cytotoxic effector cells in preparations of adherent mononuclear cells from human peripheral blood. J Immunol. 1984 Mar;132(3):1255–1260. [PubMed] [Google Scholar]
- Haynes B. F., Hemler M., Cotner T., Mann D. L., Eisenbarth G. S., Strominger J. L., Fauci A. S. Characterization of a monoclonal antibody (5E9) that defines a human cell surface antigen of cell activation. J Immunol. 1981 Jul;127(1):347–351. [PubMed] [Google Scholar]
- Hibbs M. S., Postlethwaite A. E., Mainardi C. L., Seyer J. M., Kang A. H. Alterations in collagen production in mixed mononuclear leukocyte-fibroblast cultures. J Exp Med. 1983 Jan 1;157(1):47–59. doi: 10.1084/jem.157.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoshita T., Whiteside T. L., Rodnan G. P., Taylor F. H. Abnormalities of T lymphocyte subsets in patients with progressive systemic sclerosis (PSS, scleroderma). J Lab Clin Med. 1981 Feb;97(2):264–277. [PubMed] [Google Scholar]
- Jimenez S. A., McArthur W., Rosenbloom J. Inhibition of collagen synthesis by mononuclear cell supernates. J Exp Med. 1979 Dec 1;150(6):1421–1431. doi: 10.1084/jem.150.6.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R. L., Ziff M. Lymphokine stimulation of collagen accumulation. J Clin Invest. 1976 Jul;58(1):240–252. doi: 10.1172/JCI108455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keystone E. C., Lau C., Gladman D. D., Wilkinson S., Lee P., Shore A. Immunoregulatory T cell subpopulations in patients with scleroderma using monoclonal antibodies. Clin Exp Immunol. 1982 May;48(2):443–448. [PMC free article] [PubMed] [Google Scholar]
- Lipsky P. E. Immunosuppression by D-penicillamine in vitro. Inhibition of human T lymphocyte proliferation by copper- or ceruloplasmin-dependent generation of hydrogen peroxide and protection by monocytes. J Clin Invest. 1984 Jan;73(1):53–65. doi: 10.1172/JCI111207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrack P., Endres R., Shimonkevitz R., Zlotnik A., Dialynas D., Fitch F., Kappler J. The major histocompatibility complex-restricted antigen receptor on T cells. II. Role of the L3T4 product. J Exp Med. 1983 Oct 1;158(4):1077–1091. doi: 10.1084/jem.158.4.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moroi Y., Peebles C., Fritzler M. J., Steigerwald J., Tan E. M. Autoantibody to centromere (kinetochore) in scleroderma sera. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1627–1631. doi: 10.1073/pnas.77.3.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimni M. E., Bavetta L. A. Collagen defect induced by penicillamine. Science. 1965 Nov 12;150(3698):905–907. doi: 10.1126/science.150.3698.905. [DOI] [PubMed] [Google Scholar]
- Perussia B., Acuto O., Terhorst C., Faust J., Lazarus R., Fanning V., Trinchieri G. Human natural killer cells analyzed by B73.1, a monoclonal antibody blocking Fc receptor functions. II. Studies of B73.1 antibody-antigen interaction on the lymphocyte membrane. J Immunol. 1983 May;130(5):2142–2148. [PubMed] [Google Scholar]
- Perussia B., Fanning V., Trinchieri G. A human NK and K cell subset shares with cytotoxic T cells expression of the antigen recognized by antibody OKT8. J Immunol. 1983 Jul;131(1):223–231. [PubMed] [Google Scholar]
- Perussia B., Starr S., Abraham S., Fanning V., Trinchieri G. Human natural killer cells analyzed by B73.1, a monoclonal antibody blocking Fc receptor functions. I. Characterization of the lymphocyte subset reactive with B73.1. J Immunol. 1983 May;130(5):2133–2141. [PubMed] [Google Scholar]
- Perussia B., Trinchieri G., Jackson A., Warner N. L., Faust J., Rumpold H., Kraft D., Lanier L. L. The Fc receptor for IgG on human natural killer cells: phenotypic, functional, and comparative studies with monoclonal antibodies. J Immunol. 1984 Jul;133(1):180–189. [PubMed] [Google Scholar]
- Postlethwaite A. E., Smith G. N., Mainardi C. L., Seyer J. M., Kang A. H. Lymphocyte modulation of fibroblast function in vitro: stimulation and inhibition of collagen production by different effector molecules. J Immunol. 1984 May;132(5):2470–2477. [PubMed] [Google Scholar]
- Potter S. R., Bienenstock J., Lee P., Wilkinson S., Buchanan W. W. Clinical associations of fibroblast growth promoting factor in scleroderma. J Rheumatol. 1984 Feb;11(1):43–47. [PubMed] [Google Scholar]
- ROPES M. W., BENNETT G. A., COBB S., JACOX R., JESSAR R. A. 1958 Revision of diagnostic criteria for rheumatoid arthritis. Bull Rheum Dis. 1958 Dec;9(4):175–176. [PubMed] [Google Scholar]
- Rosenbloom J., Feldman G., Freundlich B., Jimenez S. A. Transcriptional control of human diploid fibroblast collagen synthesis by gamma-interferon. Biochem Biophys Res Commun. 1984 Aug 30;123(1):365–372. doi: 10.1016/0006-291x(84)90422-4. [DOI] [PubMed] [Google Scholar]
- Rothfield N. F., Rodnan G. P. Serum antinuclear antibodies in progressive systemic sclerosis (scleroderma). Arthritis Rheum. 1968 Oct;11(5):607–617. doi: 10.1002/art.1780110502. [DOI] [PubMed] [Google Scholar]
- Roumm A. D., Whiteside T. L., Medsger T. A., Jr, Rodnan G. P. Lymphocytes in the skin of patients with progressive systemic sclerosis. Quantification, subtyping, and clinical correlations. Arthritis Rheum. 1984 Jun;27(6):645–653. doi: 10.1002/art.1780270607. [DOI] [PubMed] [Google Scholar]
- Stashenko P., Nadler L. M., Hardy R., Schlossman S. F. Characterization of a human B lymphocyte-specific antigen. J Immunol. 1980 Oct;125(4):1678–1685. [PubMed] [Google Scholar]
- Steen V. D., Medsger T. A., Jr, Rodnan G. P. D-Penicillamine therapy in progressive systemic sclerosis (scleroderma): a retrospective analysis. Ann Intern Med. 1982 Nov;97(5):652–659. doi: 10.7326/0003-4819-97-5-652. [DOI] [PubMed] [Google Scholar]
- Uchiyama T., Broder S., Waldmann T. A. A monoclonal antibody (anti-Tac) reactive with activated and functionally mature human T cells. I. Production of anti-Tac monoclonal antibody and distribution of Tac (+) cells. J Immunol. 1981 Apr;126(4):1393–1397. [PubMed] [Google Scholar]
- Whiteside T. L., Kumagai Y., Roumm A. D., Almendinger R., Rodnan G. P. Suppressor cell function and T lymphocyte subpopulations in peripheral blood of patients with progressive systemic sclerosis. Arthritis Rheum. 1983 Jul;26(7):841–847. doi: 10.1002/art.1780260704. [DOI] [PubMed] [Google Scholar]
- Yu D. T., Winchester R. J., Fu S. M., Gibofsky A., Ko H. S., Kunkel H. G. Peripheral blood Ia-positive T cells. Increases in certain diseases and after immunization. J Exp Med. 1980 Jan 1;151(1):91–100. doi: 10.1084/jem.151.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]


