Abstract
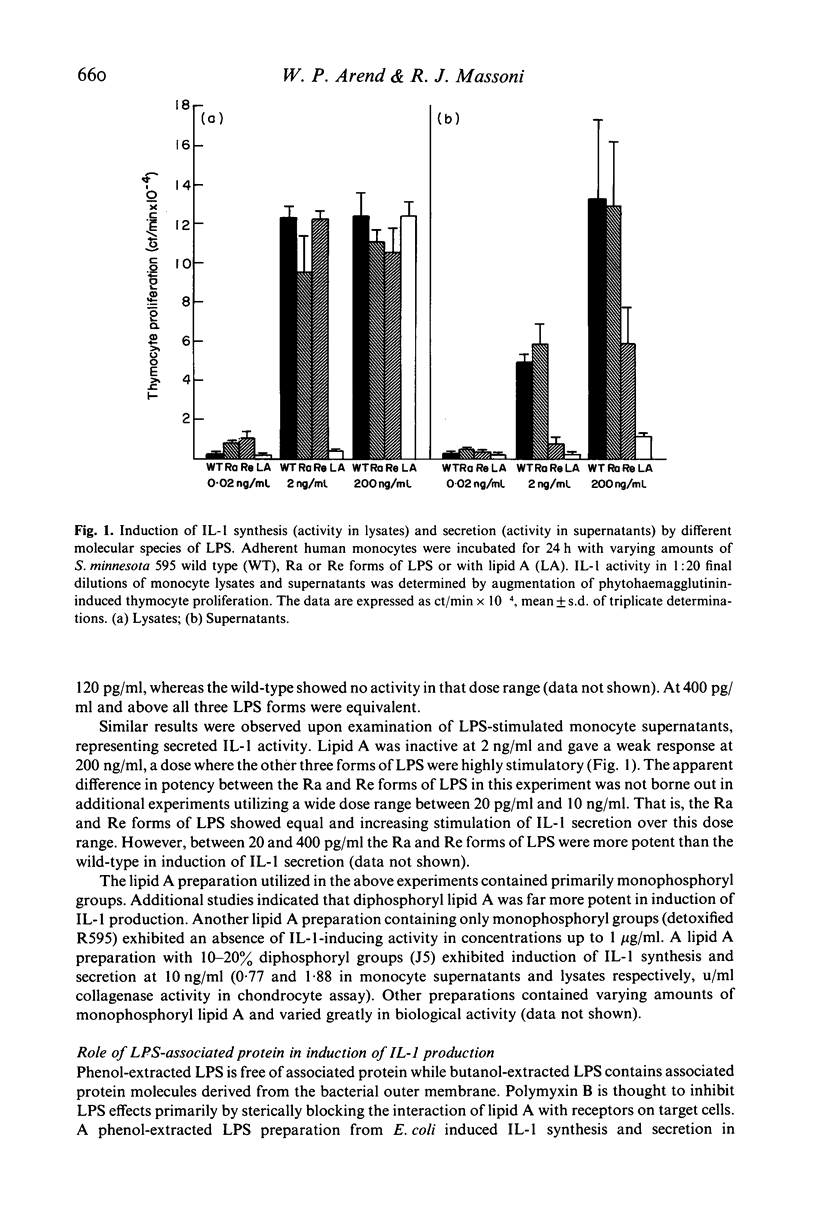
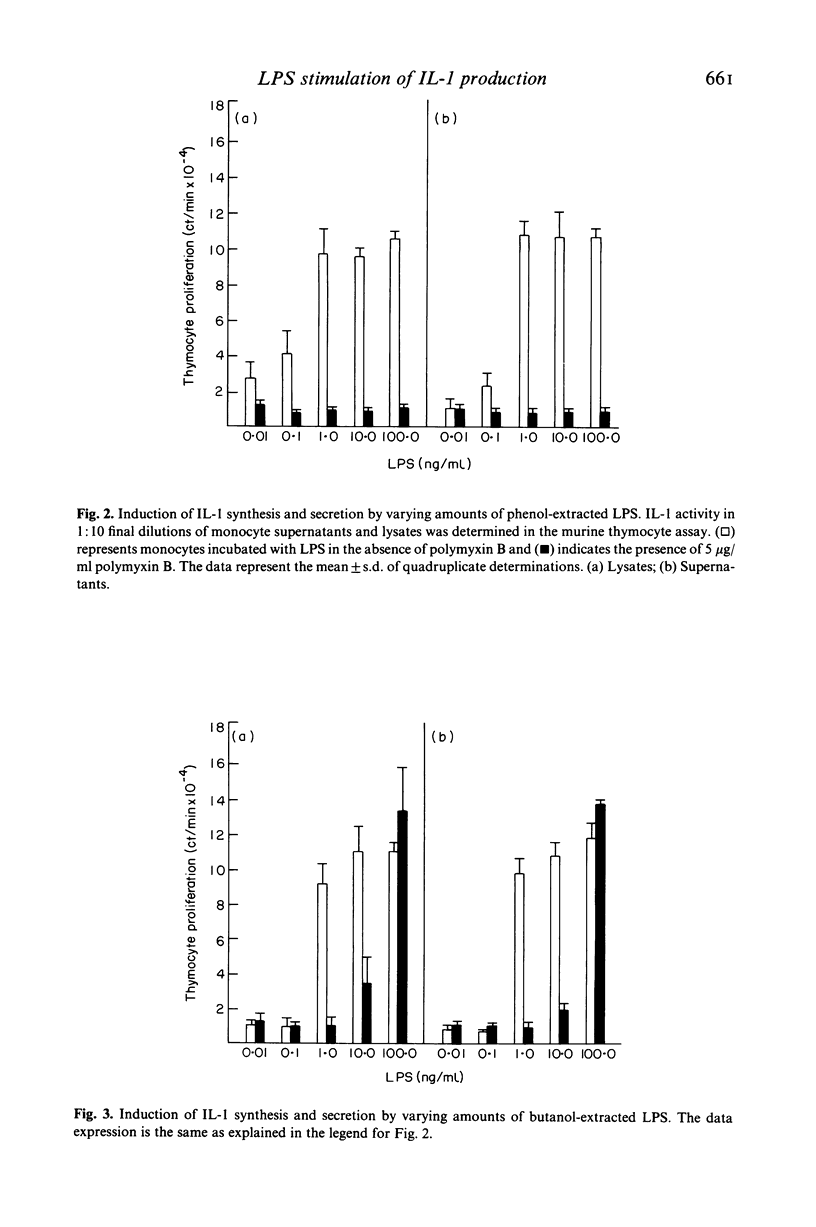
The objective of these studies was to characterize some aspects of interleukin 1 (IL-1) synthesis and secretion by human monocytes after stimulation with bacterial lipopolysaccharides (LPS). Various molecular species of LPS were incubated with adherent monocytes for 24 h. IL-1 activity in monocyte supernatants (secretion) and lysates (synthesis) was determined by stimulation of collagenase production in rabbit articular chondrocytes and augmentation of mitogen-induced proliferation of murine thymocytes. The presence of cytochalasin B enhanced LPS-induced IL-1 secretion without altering IL-1 synthesis. Monocytes preincubated in dexamethasone or hydrocortisone failed to exhibit any IL-1 activity in supernatants after LPS stimulation but the cell lysates still possessed 50% of control IL-1 activity. Studies with different LPS preparations indicated that the presence of diphosphoryl groups in lipid A enhanced the IL-1-inducing activities. Butanol-extracted LPS preparations, containing associated proteins, were not completely inhibited by 5 micrograms/ml polymyxin B in induction of IL-1 production at LPS concentrations of 10 or 100 ng/ml. These results indicate that the failure of polymyxin B to inhibit stimulation of IL-1 production by tests materials cannot be assumed to mean an absence of contaminating LPS.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arend W. P., Joslin F. G., Massoni R. J. Characteristics of chondrocyte responses to a human interleukin 1-like factor. Clin Immunol Immunopathol. 1985 Sep;36(3):358–370. doi: 10.1016/0090-1229(85)90056-x. [DOI] [PubMed] [Google Scholar]
- Arend W. P., Joslin F. G., Massoni R. J. Effects of immune complexes on production by human monocytes of interleukin 1 or an interleukin 1 inhibitor. J Immunol. 1985 Jun;134(6):3868–3875. [PubMed] [Google Scholar]
- Arend W. P., Massoni R. J. The effect of complement in adherent immune complexes on Fc and C3 receptor expression in human monocytes. Immunology. 1981 Dec;44(4):717–725. [PMC free article] [PubMed] [Google Scholar]
- Arnold A., Lipkowitz S., Suthanthiran M., Novogrodsky A., Stenzel K. H. Human B lymphoblastoid cell lines provide an interleukin 1-like signal for mitogen-treated T lymphocytes via direct cell contact. J Immunol. 1985 Jun;134(6):3876–3881. [PubMed] [Google Scholar]
- Daley L., Pier G. B., Liporace J. D., Eardley D. D. Polyclonal B cell stimulation and interleukin 1 induction by the mucoid exopolysaccharide of Pseudomonas aeruginosa associated with cystic fibrosis. J Immunol. 1985 May;134(5):3089–3093. [PubMed] [Google Scholar]
- Dinarello C. A. Interleukin-1. Rev Infect Dis. 1984 Jan-Feb;6(1):51–95. doi: 10.1093/clinids/6.1.51. [DOI] [PubMed] [Google Scholar]
- Doe W. F., Yang S. T., Morrison D. C., Betz S. J., Henson P. M. Macrophage stimulation by bacterial lipopolysaccharides. II. Evidence for differentiation signals delivered by lipid A and by a protein rich fraction of lipopolysaccharides. J Exp Med. 1978 Aug 1;148(2):557–568. doi: 10.1084/jem.148.2.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haeffner-Cavaillon N., Cavaillon J. M., Moreau M., Szabó L. Interleukin 1 secretion by human monocytes stimulated by the isolated polysaccharide region of the Bordetella pertussis endotoxin. Mol Immunol. 1984 May;21(5):389–395. doi: 10.1016/0161-5890(84)90036-1. [DOI] [PubMed] [Google Scholar]
- Kido N., Nakashima I., Kato N. Correlation between strong adjuvanticity of Klebsiella O3 lipopolysaccharide and its ability to induce interleukin-1 secretion. Cell Immunol. 1984 May;85(2):477–486. doi: 10.1016/0008-8749(84)90260-0. [DOI] [PubMed] [Google Scholar]
- Kido N., Ohta M., Ito H., Naito S., Nagase F., Nakashima I., Kato N. Potent adjuvant action of lipopolysaccharides possessing the O-specific polysaccharide moieties consisting of mannans in antibody response against protein antigen. Cell Immunol. 1985 Mar;91(1):52–59. doi: 10.1016/0008-8749(85)90031-0. [DOI] [PubMed] [Google Scholar]
- Kim Y. B., Watson D. W. Biologically active endotoxins from Salmonella mutants deficient in O- and R-polysaccharides and heptose. J Bacteriol. 1967 Nov;94(5):1320–1326. doi: 10.1128/jb.94.5.1320-1326.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurt-Jones E. A., Beller D. I., Mizel S. B., Unanue E. R. Identification of a membrane-associated interleukin 1 in macrophages. Proc Natl Acad Sci U S A. 1985 Feb;82(4):1204–1208. doi: 10.1073/pnas.82.4.1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepe-Zuniga J. L., Gery I. Production of intra- and extracellular interleukin-1 (IL-1) by human monocytes. Clin Immunol Immunopathol. 1984 May;31(2):222–230. doi: 10.1016/0090-1229(84)90242-3. [DOI] [PubMed] [Google Scholar]
- Mizel S. B., Oppenheim J. J., Rosenstreich D. L. Characterization of lymphocyte-activating factor (LAF) produced by the macrophage cell line, P388D1. I. Enhancement of LAF production by activated T lymphocytes. J Immunol. 1978 May;120(5):1497–1503. [PubMed] [Google Scholar]
- Morrison D. C., Betz S. J. Chemical and biological properties of a protein-rich fraction of bacterial lipopolysaccharides. II. The in vitro rat peritoneal mast cell response. J Immunol. 1977 Nov;119(5):1790–1795. [PubMed] [Google Scholar]
- Morrison D. C., Betz S. J., Jacobs D. M. Isolation of a lipid A bound polypeptide responsible for "LPS-initiated" mitogenesis of C3H/HeJ spleen cells. J Exp Med. 1976 Sep 1;144(3):840–846. doi: 10.1084/jem.144.3.840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy P. A., Simon P. L., Willoughby W. F. Endogenous pyrogens made by rabbit peritoneal exudate cells are identical with lymphocyte-activating factors made by rabbit alveolar macrophages. J Immunol. 1980 May;124(5):2498–2501. [PubMed] [Google Scholar]
- Nishijima M., Amano F., Akamatsu Y., Akagawa K., Tokunaga T., Raetz C. R. Macrophage activation by monosaccharide precursors of Escherichia coli lipid A. Proc Natl Acad Sci U S A. 1985 Jan;82(2):282–286. doi: 10.1073/pnas.82.2.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raetz C. R., Purcell S., Takayama K. Molecular requirements for B-lymphocyte activation by Escherichia coli lipopolysaccharide. Proc Natl Acad Sci U S A. 1983 Aug;80(15):4624–4628. doi: 10.1073/pnas.80.15.4624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribi E. Beneficial modification of the endotoxin molecule. J Biol Response Mod. 1984;3(1):1–9. [PubMed] [Google Scholar]
- Stosić-Grujicić S., Simić M. M. Modulation of interleukin 1 production by activated macrophages: in vitro action of hydrocortisone, colchicine, and cytochalasin B. Cell Immunol. 1982 May 15;69(2):235–247. doi: 10.1016/0008-8749(82)90070-3. [DOI] [PubMed] [Google Scholar]
- Van Lenten B. J., Fogelman A. M., Seager J., Ribi E., Haberland M. E., Edwards P. A. Bacterial endotoxin selectively prevents the expression of scavenger-receptor activity on human monocyte-macrophages. J Immunol. 1985 Jun;134(6):3718–3721. [PubMed] [Google Scholar]
- Williamson S. I., Wannemuehler M. J., Jirillo E., Pritchard D. G., Michalek S. M., McGhee J. R. LPS regulation of the immune response: separate mechanisms for murine B cell activation by lipid A (direct) and polysaccharide (macrophage-dependent) derived from Bacteroides LPS. J Immunol. 1984 Nov;133(5):2294–2300. [PubMed] [Google Scholar]
- Wood D. D., Bayne E. K., Goldring M. B., Gowen M., Hamerman D., Humes J. L., Ihrie E. J., Lipsky P. E., Staruch M. J. The four biochemically distinct species of human interleukin 1 all exhibit similar biologic activities. J Immunol. 1985 Feb;134(2):895–903. [PubMed] [Google Scholar]


