Abstract
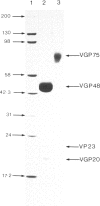
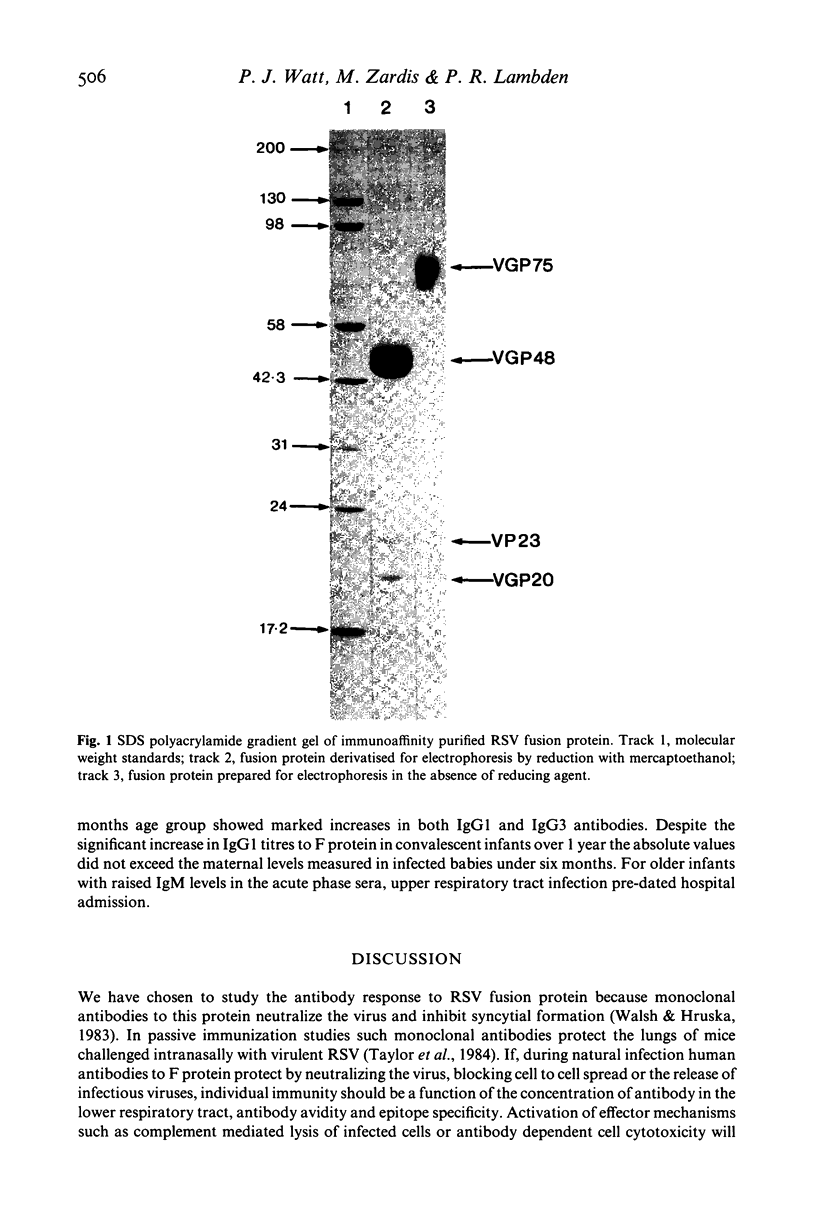
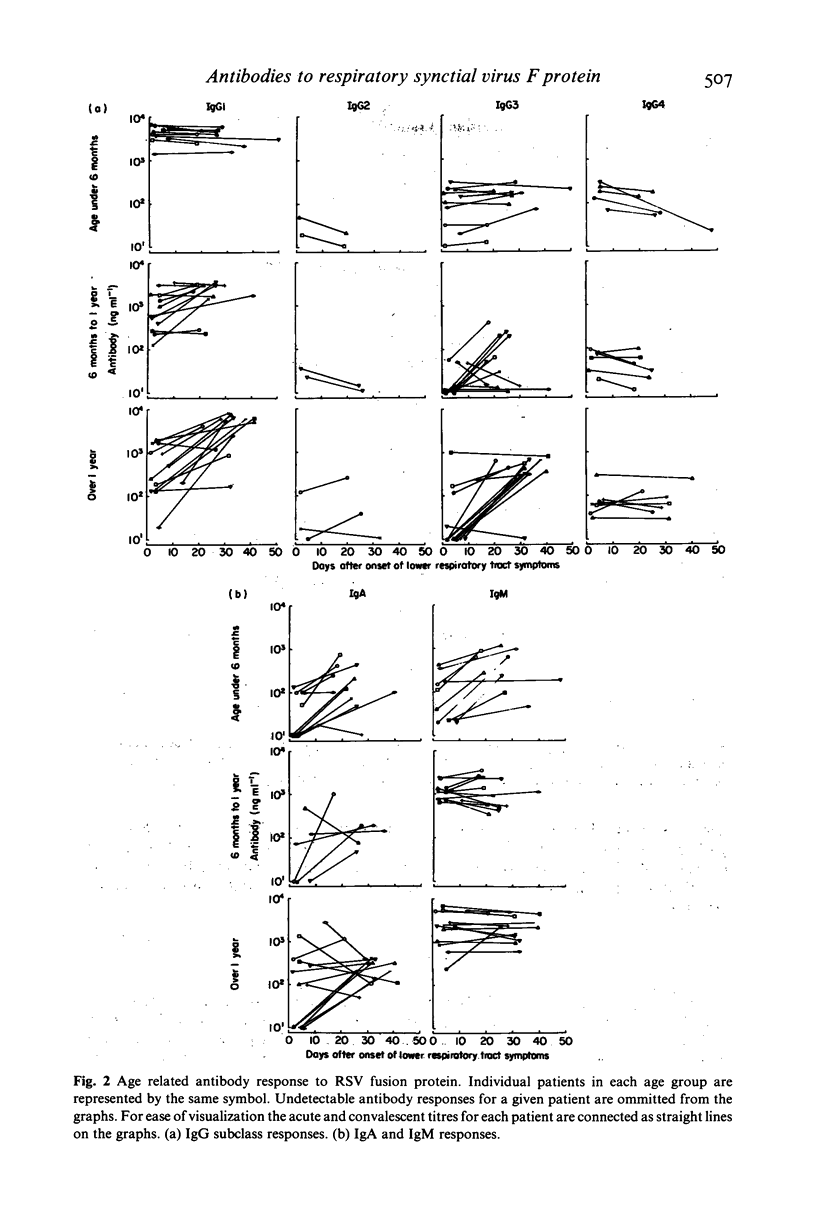
Respiratory syncytial virus (RSV) fusion protein was purified by immunoaffinity chromatography using a mouse monoclonal antibody coupled to Affi-gel 10. The fusion protein was homogeneous by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and free of other detectable viral or cellular protein. The purified fusion protein was used in a quantitative enzyme-linked immunosorbent assay (ELISA) to measure the age-related antibody response to this protein in infected infants. The four IgG subclasses, IgA and IgM levels were determined for infants under 6 months of age, infants aged 6 months to 1 year and infants aged 1 year and over. Most infants over 6 months of age showed marked increases in both IgG1 and IgG3 antibodies with poor or negligible response with IgG2 and IgG4. By contrast infants under 6 months failed to respond by the production of IgG antibodies, although increases in IgA and IgM levels were observed. These data may explain the failure of primary RSV infections to induce protective immunity and have implications for the strategic use of attenuated RSV vaccines.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bell D. Y., Haseman J. A., Spock A., McLennan G., Hook G. E. Plasma proteins of the bronchoalveolar surface of the lungs of smokers and nonsmokers. Am Rev Respir Dis. 1981 Jul;124(1):72–79. doi: 10.1164/arrd.1981.124.1.72. [DOI] [PubMed] [Google Scholar]
- Belshe R. B., Van Voris L. P., Mufson M. A., Buynak E. B., McLean A. A., Hilleman M. A. Comparison of enzyme-linked immunosorbent assay and neutralization techniques for measurement of antibody to respiratory syncytial virus: implications for parenteral immunization with live virus vaccine. Infect Immun. 1982 Jul;37(1):160–165. doi: 10.1128/iai.37.1.160-165.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman R. M., Nahmias A. J., Williams S. C., Phillips D. J., Black C. M., Reimer C. B. IgG subclass antibodies to herpes simplex virus. J Infect Dis. 1985 May;151(5):929–936. doi: 10.1093/infdis/151.5.929. [DOI] [PubMed] [Google Scholar]
- Farrar W. E., Jr Molecular analysis of plasmids in epidemiologic investigation. J Infect Dis. 1983 Jul;148(1):1–6. doi: 10.1093/infdis/148.1.1. [DOI] [PubMed] [Google Scholar]
- Fulginiti V. A., Eller J. J., Sieber O. F., Joyner J. W., Minamitani M., Meiklejohn G. Respiratory virus immunization. I. A field trial of two inactivated respiratory virus vaccines; an aqueous trivalent parainfluenza virus vaccine and an alum-precipitated respiratory syncytial virus vaccine. Am J Epidemiol. 1969 Apr;89(4):435–448. doi: 10.1093/oxfordjournals.aje.a120956. [DOI] [PubMed] [Google Scholar]
- Glezen W. P., Paredes A., Allison J. E., Taber L. H., Frank A. L. Risk of respiratory syncytial virus infection for infants from low-income families in relationship to age, sex, ethnic group, and maternal antibody level. J Pediatr. 1981 May;98(5):708–715. doi: 10.1016/s0022-3476(81)80829-3. [DOI] [PubMed] [Google Scholar]
- Henderson F. W., Collier A. M., Clyde W. A., Jr, Denny F. W. Respiratory-syncytial-virus infections, reinfections and immunity. A prospective, longitudinal study in young children. N Engl J Med. 1979 Mar 8;300(10):530–534. doi: 10.1056/NEJM197903083001004. [DOI] [PubMed] [Google Scholar]
- Kim H. W., Canchola J. G., Brandt C. D., Pyles G., Chanock R. M., Jensen K., Parrott R. H. Respiratory syncytial virus disease in infants despite prior administration of antigenic inactivated vaccine. Am J Epidemiol. 1969 Apr;89(4):422–434. doi: 10.1093/oxfordjournals.aje.a120955. [DOI] [PubMed] [Google Scholar]
- Linde G. A. Subclass distribution of rubella virus-specific immunoglobulin G. J Clin Microbiol. 1985 Jan;21(1):117–121. doi: 10.1128/jcm.21.1.117-121.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrill W. W., Naegel G. P., Olchowski J. J., Reynolds H. Y. Immunoglobulin G subclass proteins in serum and lavage fluid of normal subjects. Quantitation and comparison with immunoglobulins A and E. Am Rev Respir Dis. 1985 Apr;131(4):584–587. doi: 10.1164/arrd.1985.131.4.584. [DOI] [PubMed] [Google Scholar]
- Naegel G. P., Young K. R., Jr, Reynolds H. Y. Receptors for human IgG subclasses on human alveolar macrophages. Am Rev Respir Dis. 1984 Mar;129(3):413–418. doi: 10.1164/arrd.1984.129.3.413. [DOI] [PubMed] [Google Scholar]
- Ogilvie M. M., Vathenen A. S., Radford M., Codd J., Key S. Maternal antibody and respiratory syncytial virus infection in infancy. J Med Virol. 1981;7(4):263–271. doi: 10.1002/jmv.1890070403. [DOI] [PubMed] [Google Scholar]
- Oxelius V. A. IgG subclass levels in infancy and childhood. Acta Paediatr Scand. 1979 Jan;68(1):23–27. doi: 10.1111/j.1651-2227.1979.tb04424.x. [DOI] [PubMed] [Google Scholar]
- Parrott R. H., Kim H. W., Arrobio J. O., Hodes D. S., Murphy B. R., Brandt C. D., Camargo E., Chanock R. M. Epidemiology of respiratory syncytial virus infection in Washington, D.C. II. Infection and disease with respect to age, immunologic status, race and sex. Am J Epidemiol. 1973 Oct;98(4):289–300. doi: 10.1093/oxfordjournals.aje.a121558. [DOI] [PubMed] [Google Scholar]
- Prince G. A., Horswood R. L., Camargo E., Suffin S. C., Chanock R. M. Parenteral immunization with live respiratory syncytial virus is blocked in seropositive cotton rats. Infect Immun. 1982 Sep;37(3):1074–1078. doi: 10.1128/iai.37.3.1074-1078.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skvaril F., Schilt U. Characterization of the subclasses and light chain types of IgG antibodies to rubella. Clin Exp Immunol. 1984 Mar;55(3):671–676. [PMC free article] [PubMed] [Google Scholar]
- Sundqvist V. A., Linde A., Wahren B. Virus-specific immunoglobulin G subclasses in herpes simplex and varicella-zoster virus infections. J Clin Microbiol. 1984 Jul;20(1):94–98. doi: 10.1128/jcm.20.1.94-98.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor G., Stott E. J., Bew M., Fernie B. F., Cote P. J., Collins A. P., Hughes M., Jebbett J. Monoclonal antibodies protect against respiratory syncytial virus infection in mice. Immunology. 1984 May;52(1):137–142. [PMC free article] [PubMed] [Google Scholar]
- Walsh E. E., Hruska J. Monoclonal antibodies to respiratory syncytial virus proteins: identification of the fusion protein. J Virol. 1983 Jul;47(1):171–177. doi: 10.1128/jvi.47.1.171-177.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward K. A., Lambden P. R., Ogilvie M. M., Watt P. J. Antibodies to respiratory syncytial virus polypeptides and their significance in human infection. J Gen Virol. 1983 Sep;64(Pt 9):1867–1876. doi: 10.1099/0022-1317-64-9-1867. [DOI] [PubMed] [Google Scholar]