Abstract
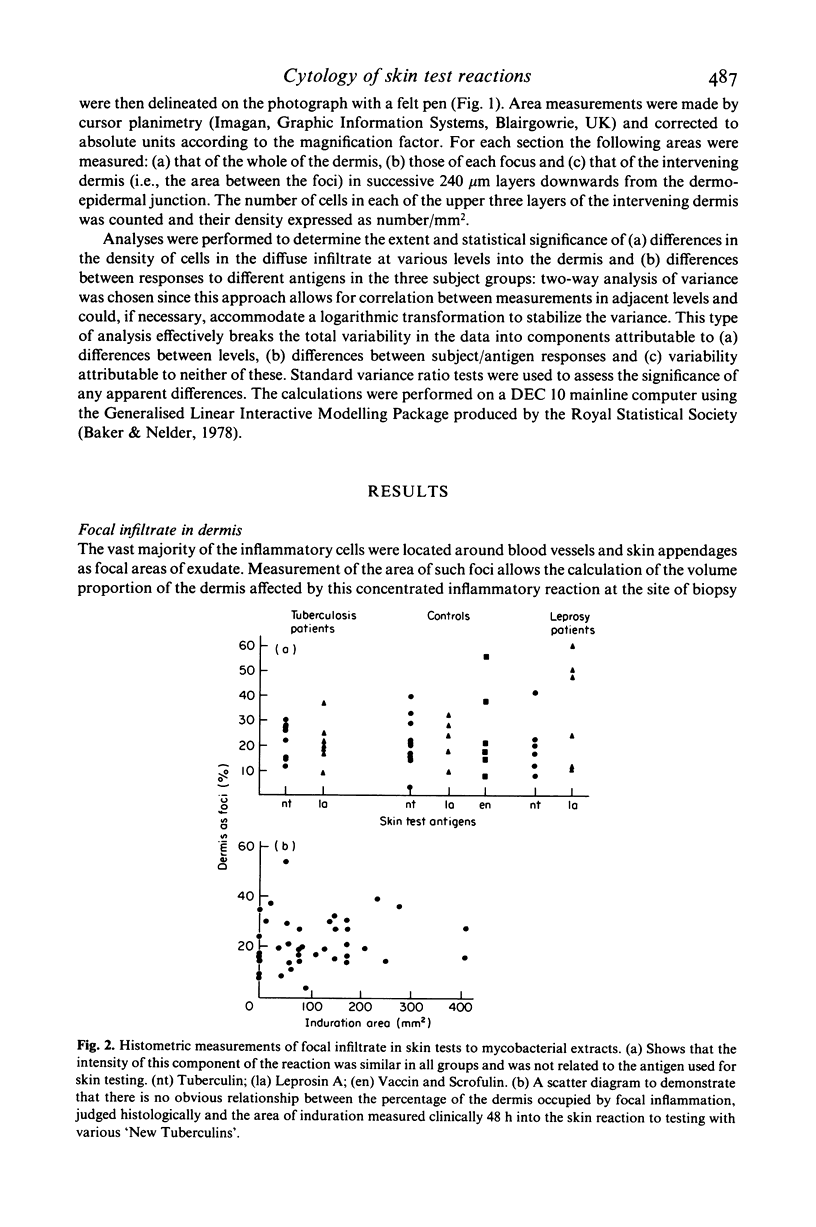
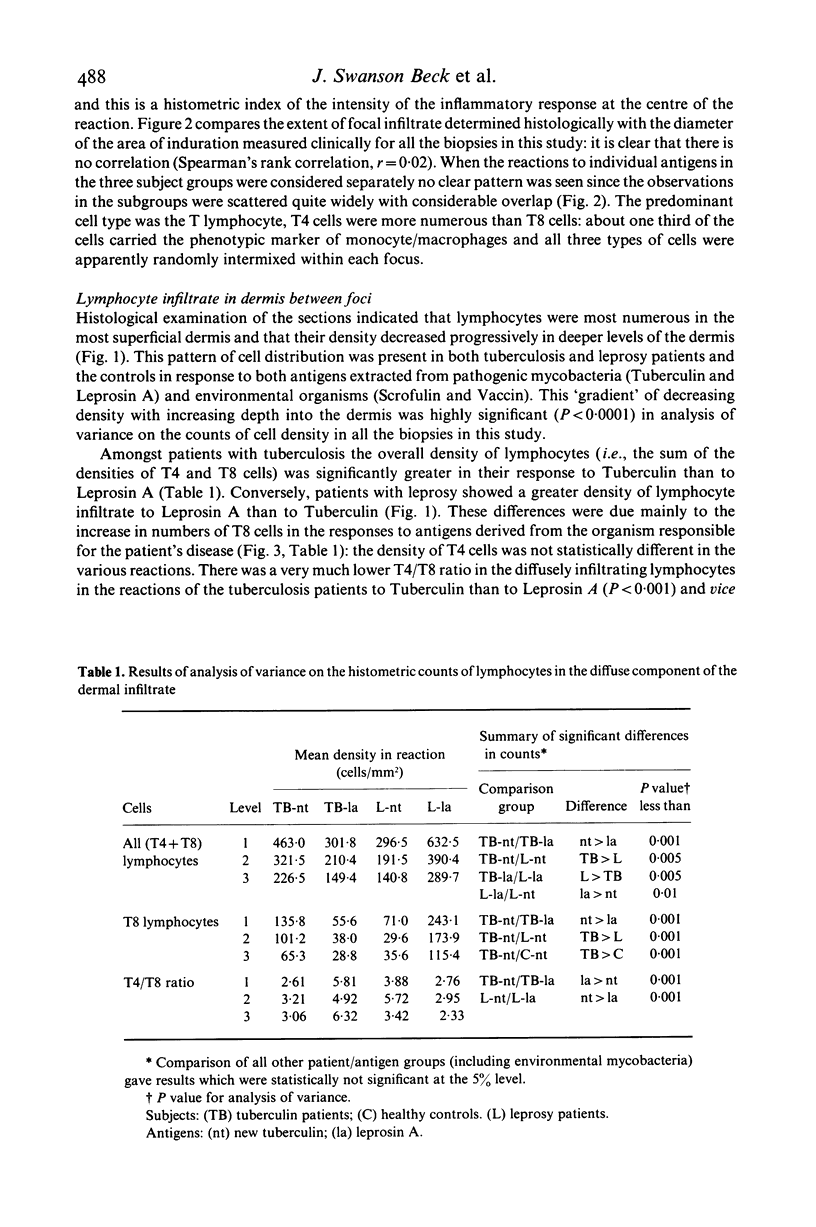
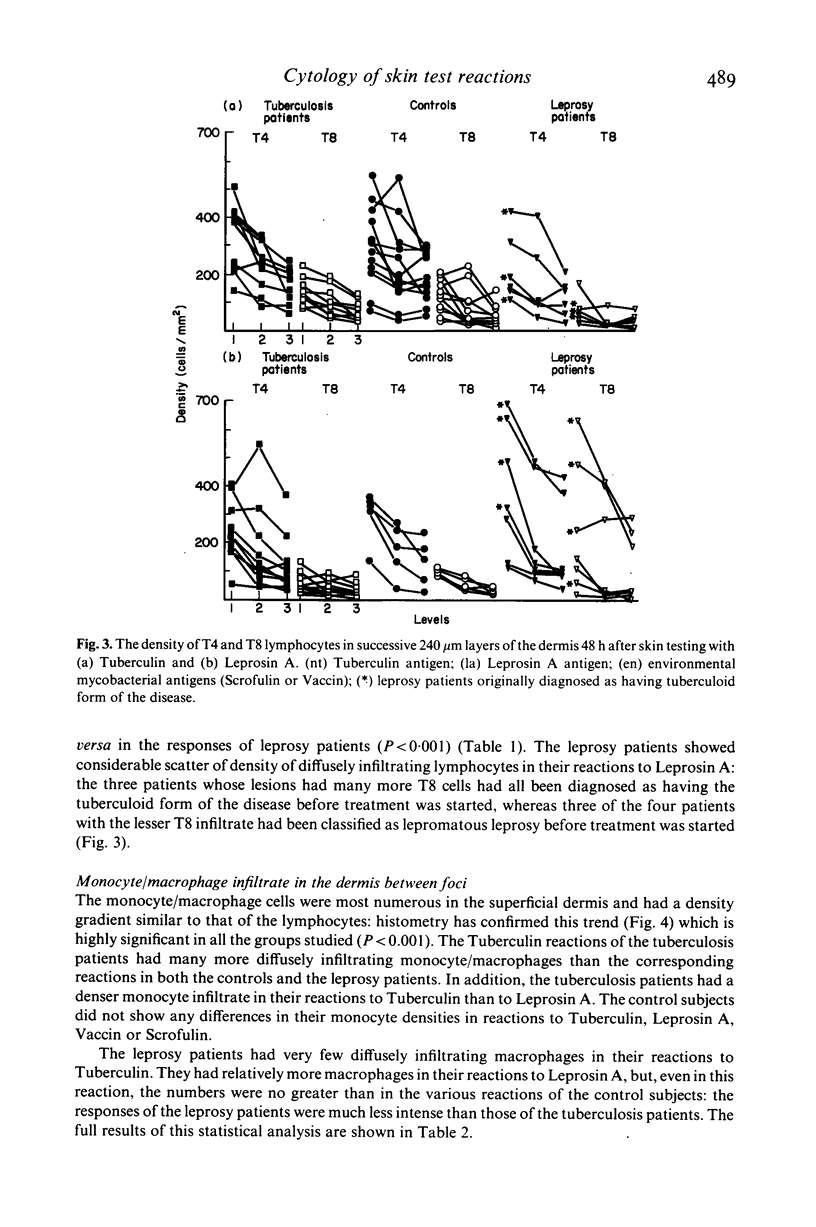
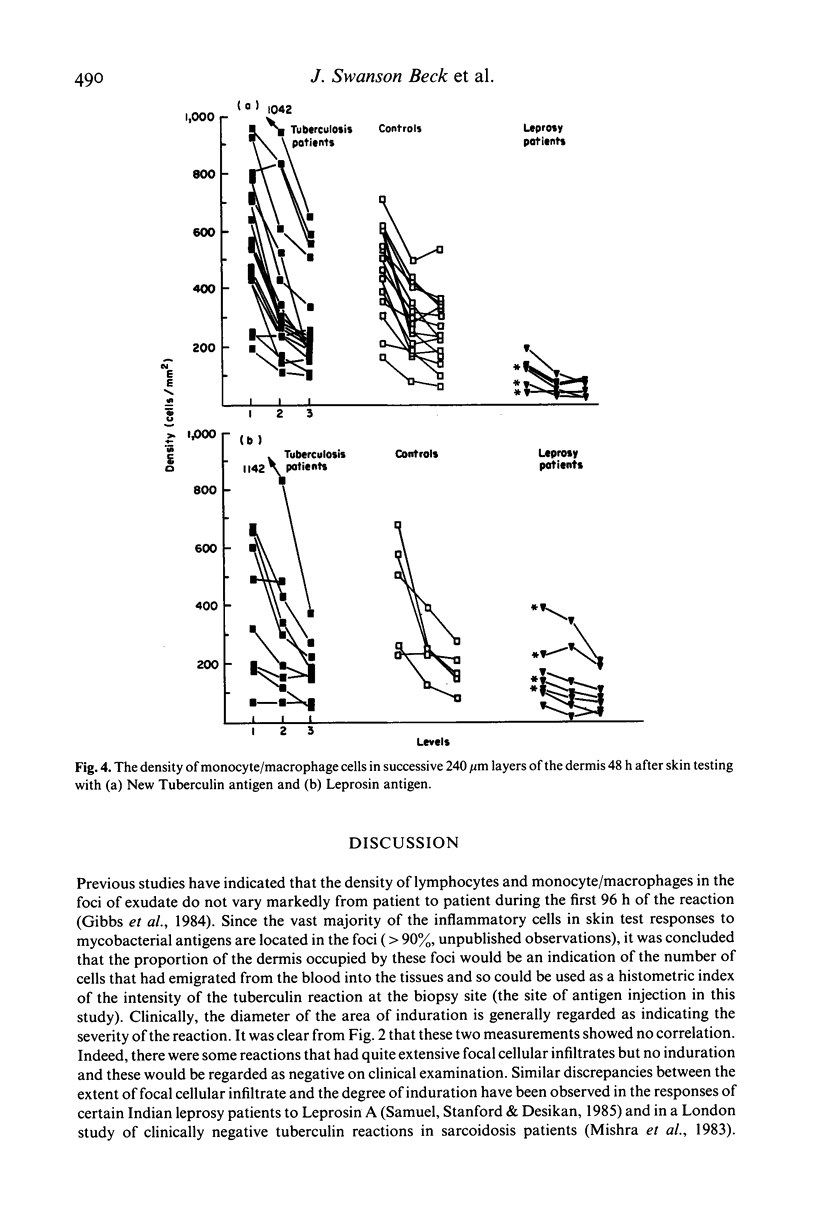
The density and distribution of T4 and T8 lymphocytes and of monocyte/macrophages at the site of skin tests with mycobacterial antigens was studied in pulmonary tuberculosis and leprosy patients and in healthy controls. Most of the inflammatory cells were located in perivascular and periappendicular foci in the dermis: the percentage of the dermis occupied by focal infiltrate was unrelated to the clinical measurement of the area of induration. There was a less intense diffuse infiltrate in the dermis between the foci, most marked in the papillary dermis and lessening progressively in deeper layers. In patients, diffusely infiltrating lymphocytes were more numerous (mainly due to an excess of T8 cells) in relation to extracts of the pathogen causing their disease than to extracts of the other organism: T8 cells were particularly numerous in reactions to Leprosin A in three of four partly treated leprosy patients who had been classified as tuberculoid at the time of diagnosis. The density of diffusely infiltrating macrophages showed a similar density gradient and selective concentration in response to active disease pathogens. However these cells were less numerous in partly treated leprosy patients than in controls and most frequent in untreated pulmonary tuberculosis patients. Selective migration of monocyte/macrophages and, to a lesser extent T8 cells, appears to be a prominent feature in the reaction of patient with active mycobacterial disease to antigens derived from the causative organisms: this suggests that it might become possible to distinguish direct reactions from cross-reactions in human delayed hypersensitivity reactions by identification of these histological features.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLACK S., HUMPHREY J. H., NIVEN J. S. Inhibition of Mantoux reaction by direct suggestion under hypnosis. Br Med J. 1963 Jun 22;1(5346):1649–1652. doi: 10.1136/bmj.1.5346.1642-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coghill G., Gibbs J. H., Lowe J. G., Swanson Beck J. Cryopreservation with glycerol during cryostat sectioning for localisation of lymphocytes and accessory cell phenotypic subsets in tissue biopsies. J Clin Pathol. 1985 Jul;38(7):840–842. doi: 10.1136/jcp.38.7.840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel T. M., Janicki B. W. Mycobacterial antigens: a review of their isolation, chemistry, and immunological properties. Microbiol Rev. 1978 Mar;42(1):84–113. doi: 10.1128/mr.42.1.84-113.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennert G., Weiss S., Warner J. F. T cells may express multiple activities: specific allohelp, cytolysis, and delayed-type hypersensitivity are expressed by a cloned T-cell line. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4540–4543. doi: 10.1073/pnas.78.7.4540. [DOI] [PMC free article] [PubMed] [Google Scholar]
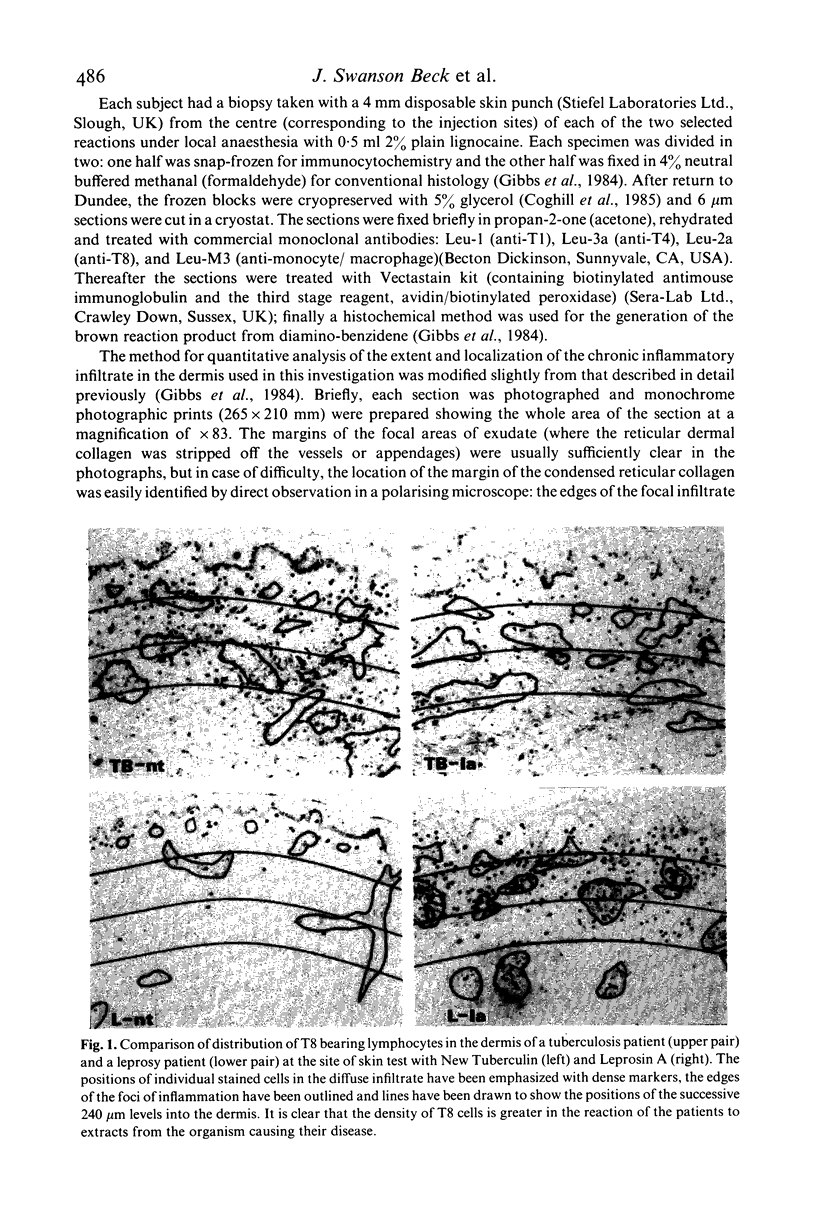
- Gibbs J. H., Ferguson J., Brown R. A., Kenicer K. J., Potts R. C., Coghill G., Swanson Beck J. Histometric study of the localisation of lymphocyte subsets and accessory cells in human Mantoux reactions. J Clin Pathol. 1984 Nov;37(11):1227–1234. doi: 10.1136/jcp.37.11.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grange J. M. The humoral immune response in tuberculosis: its nature, biological role and diagnostic usefulness. Adv Tuberc Res. 1984;21:1–78. [PubMed] [Google Scholar]
- Kardjito T., Grange J. M. Diagnosis of active tuberculosis by immunological methods. 2. Qualitative differences in the dermal response to tuberculin in patients with active pulmonary disease and healthy tuberculin-positive individuals. Tubercle. 1982 Dec;63(4):275–278. doi: 10.1016/s0041-3879(82)80015-9. [DOI] [PubMed] [Google Scholar]
- Lefford M. J. Editorial: Delayed hypersensitivity and immunity in tuberculosis. Am Rev Respir Dis. 1975 Mar;111(3):243–246. doi: 10.1164/arrd.1975.111.3.243. [DOI] [PubMed] [Google Scholar]
- Liew F. Y., Hale C., Howard J. G. Immunologic regulation of experimental cutaneous leishmaniasis. V. Characterization of effector and specific suppressor T cells. J Immunol. 1982 Apr;128(4):1917–1922. [PubMed] [Google Scholar]
- Magnusson M. Mycobacterial sensitins: where are we now? Rev Infect Dis. 1981 Sep-Oct;3(5):944–948. doi: 10.1093/clinids/3.5.944. [DOI] [PubMed] [Google Scholar]
- Mishra B. B., Poulter L. W., Janossy G., James D. G. The distribution of lymphoid and macrophage like cell subsets of sarcoid and Kveim granulomata: possible mechanism of negative PPD reaction in sarcoidosis. Clin Exp Immunol. 1983 Dec;54(3):705–715. [PMC free article] [PubMed] [Google Scholar]
- Modlin R. L., Hofman F. M., Taylor C. R., Rea T. H. T lymphocyte subsets in the skin lesions of patients with leprosy. J Am Acad Dermatol. 1983 Feb;8(2):182–189. doi: 10.1016/s0190-9622(83)70021-6. [DOI] [PubMed] [Google Scholar]
- Mustafa A. S., Godal T. In vitro induction of human suppressor T cells by mycobacterial antigens. BCG activated OKT4+ cells mediate suppression of antigen induced T cell proliferation. Clin Exp Immunol. 1983 Apr;52(1):29–37. [PMC free article] [PubMed] [Google Scholar]
- Paul R. C., Stanford J. L., Misljenóvic O., Lefering J. Multiple skin testing of Kenyan schoolchildren with a series of new tuberculins. J Hyg (Lond) 1975 Oct;75(2):303–313. doi: 10.1017/s002217240004732x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt J. L., Grant B. W., Eddy A. A., Michael A. F. Immune cell populations in cutaneous delayed-type hypersensitivity. J Exp Med. 1983 Oct 1;158(4):1227–1242. doi: 10.1084/jem.158.4.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulter L. W., Seymour G. J., Duke O., Janossy G., Panayi G. Immunohistological analysis of delayed-type hypersensitivity in man. Cell Immunol. 1982 Dec;74(2):358–369. doi: 10.1016/0008-8749(82)90036-3. [DOI] [PubMed] [Google Scholar]
- Rook G. A. Suppressor cells of mouse and man. What is the evidence that they contribute to the aetiology of the mycobacterioses? Lepr Rev. 1982 Dec;53(4):306–312. doi: 10.5935/0305-7518.19820038. [DOI] [PubMed] [Google Scholar]
- Rook G. A. The immunology of leprosy. Tubercle. 1983 Dec;64(4):297–312. doi: 10.1016/0041-3879(83)90028-4. [DOI] [PubMed] [Google Scholar]
- Samuel N. M., Stanford J. L., Desikan K. V. Microscopic findings of delayed reactions elicited by the skin test reagent Leprosin A derived from M. leprae. Int J Lepr Other Mycobact Dis. 1985 Sep;53(3):395–403. [PubMed] [Google Scholar]
- Scheynius A., Klareskog L., Forsum U. In situ identification of T lymphocyte subsets and HLA-DR expressing cells in the human skin tuberculin reaction. Clin Exp Immunol. 1982 Aug;49(2):325–330. [PMC free article] [PubMed] [Google Scholar]
- Shield M. J., Stanford J. L., Paul R. C., Carswell J. W. Multiple skin testing of tuberculosis patients with a range of new tuberculins, and a comparison with leprosy and Mycobacterium ulcerans infection. J Hyg (Lond) 1977 Jun;78(3):331–348. doi: 10.1017/s0022172400056230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanford J. L., Grange J. M. The meaning and structure of species as applied to mycobacteria. Tubercle. 1974 Jun;55(2):143–152. doi: 10.1016/0041-3879(74)90008-7. [DOI] [PubMed] [Google Scholar]
- Stanford J. L., Lema E. The use of a sonicate preparation of Mycobacterium tuberculosis (new tuberculin) in the assessment of BCG vaccination. Tubercle. 1983 Dec;64(4):275–282. doi: 10.1016/0041-3879(83)90024-7. [DOI] [PubMed] [Google Scholar]
- Stanford J. L., Nye P. M., Rook G. A., Samuel N., Fairbank A. A preliminary investigation of the responsiveness or otherwise of patients and staff of a leprosy hospital to groups of shared or species antigens of mycobacteria. Lepr Rev. 1981 Dec;52(4):321–327. doi: 10.5935/0305-7518.19810043. [DOI] [PubMed] [Google Scholar]
- Stanford J. L., Rook G. A., Convit J., Godal T., Kronvall G., Rees R. J., Walsh G. P. Preliminary taxonomic studies on the leprosy bacillus. Br J Exp Pathol. 1975 Dec;56(6):579–585. [PMC free article] [PubMed] [Google Scholar]
- Youmans G. P. Relation between delayed hypersensitivity and immunity in tuberculosis. Am Rev Respir Dis. 1975 Feb;111(2):109–118. doi: 10.1164/arrd.1975.111.2.109. [DOI] [PubMed] [Google Scholar]
- van Eden W., de Vries R. R., Stanford J. L., Rook G. A. HLA-DR3 associated genetic control of response to multiple skin tests with new tuberculins. Clin Exp Immunol. 1983 May;52(2):287–292. [PMC free article] [PubMed] [Google Scholar]