Abstract
Intravenous administration of 125I-labelled isolated mouse serum amyloid P component (SAP) to mice with systemic amyloidosis was followed by specific deposition of the labelled protein in amyloidotic organs. Although only a small proportion of the total injected dose became localized in this way, the amount correlated with the quantity of amyloid present in different organs and was greatest in the spleen. No such localization was detected in the organs of control, untreated mice or animals which had received inflammatory stimuli but did not have amyloidosis. The labelled SAP was found by autoradiography to be present in the same distribution within the tissues as the Congophilic amyloid deposits. These observations establish directly, for the first time, that circulating SAP is the precursor of the amyloid P component (AP) in systemic amyloidosis. They were confirmed by the further finding that intravenous injection into amyloidotic mice of human SAP, either in whole human serum or in isolated pure form, was followed by appearance of the human SAP in the mouse amyloid deposits. In addition to elucidating one aspect of the pathogenesis of amyloid deposition and strengthening the homology of functional behaviour between SAP of different species, the present results suggest a means for selective targeting of diagnostic tracers and/or effector agents to amyloid deposits in vivo.
Full text
PDF


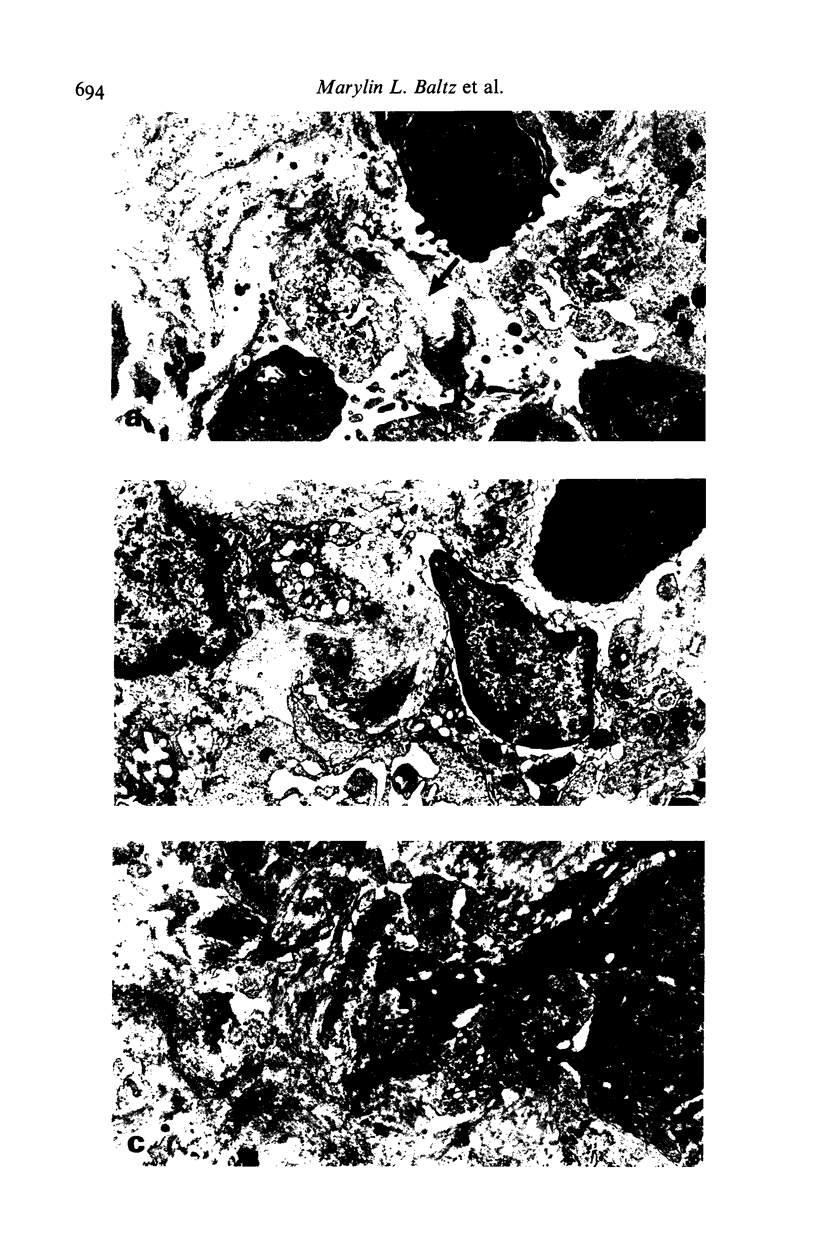
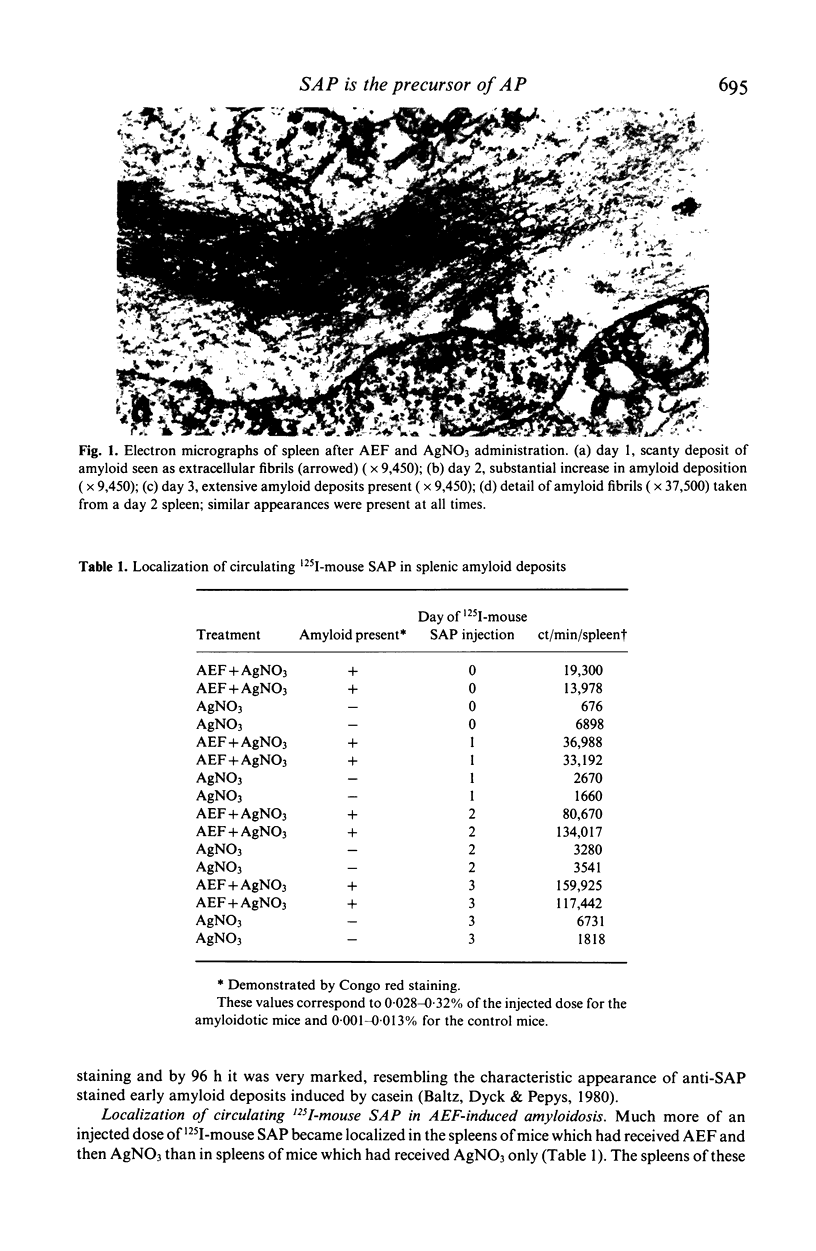
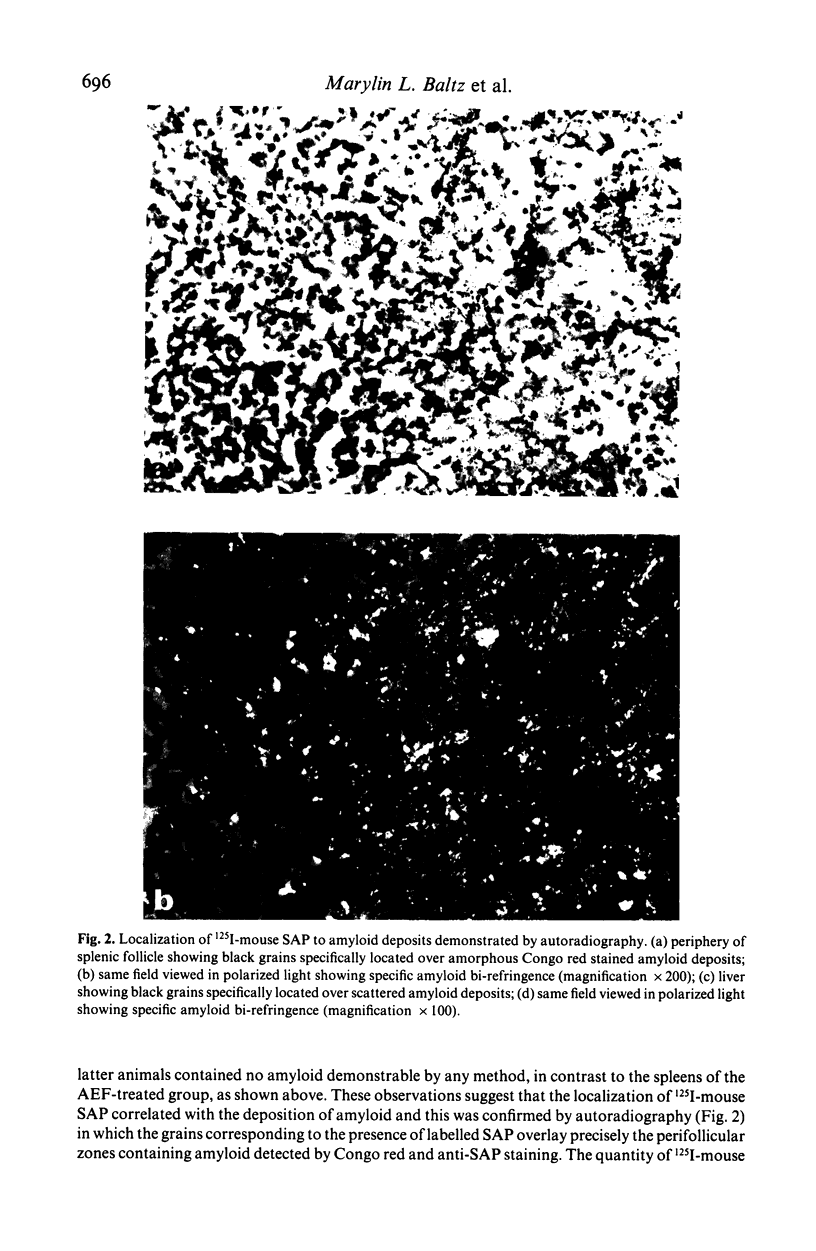
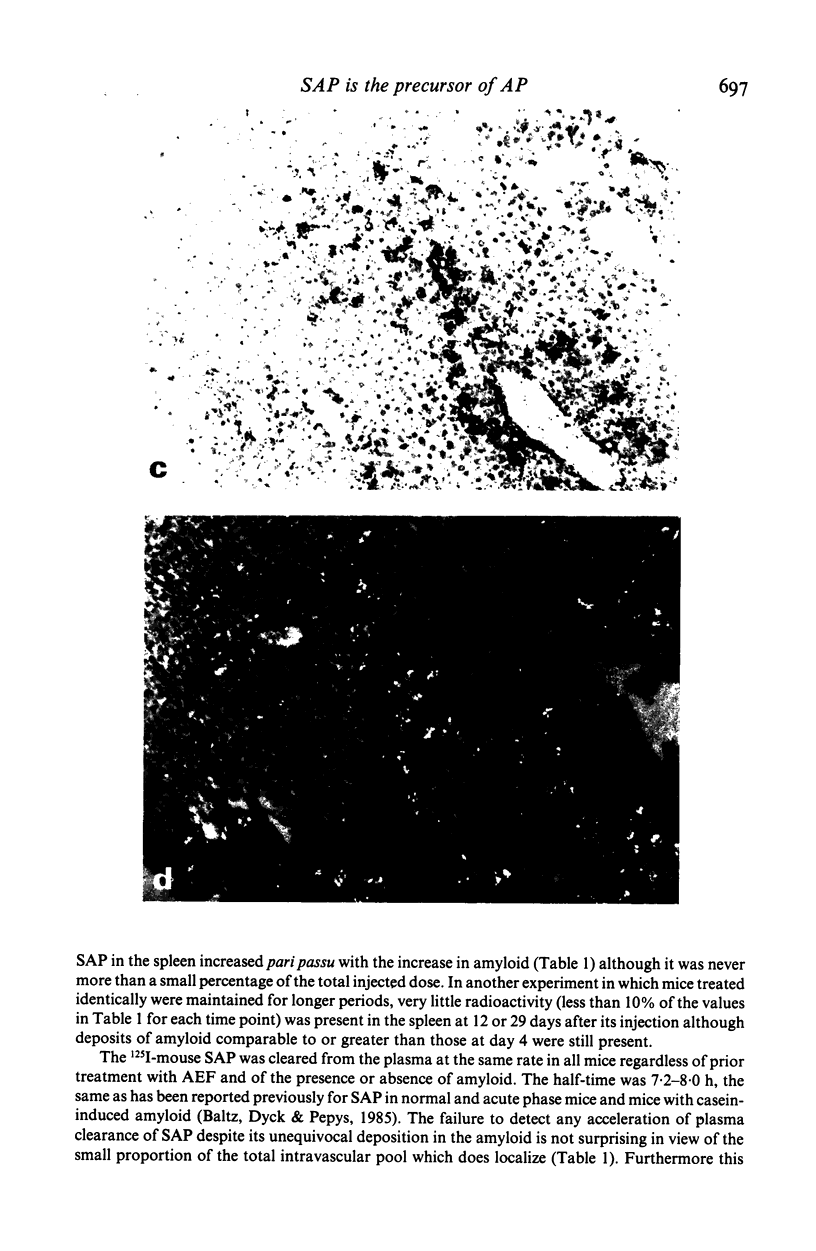


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Axelrad M. A., Kisilevsky R., Willmer J., Chen S. J., Skinner M. Further characterization of amyloid-enhancing factor. Lab Invest. 1982 Aug;47(2):139–146. [PubMed] [Google Scholar]
- Baltz M. L., Dyck R. F., Pepys M. B. Amyloid P-component in mice injected with casein: identification in amyloid deposits and in the cytoplasm of hepatocytes. Immunology. 1980 Sep;41(1):59–66. [PMC free article] [PubMed] [Google Scholar]
- Baltz M. L., de Beer F. C., Feinstein A., Munn E. A., Milstein C. P., Fletcher T. C., March J. F., Taylor J., Bruton C., Clamp J. R. Phylogenetic aspects of C-reactive protein and related proteins. Ann N Y Acad Sci. 1982;389:49–75. doi: 10.1111/j.1749-6632.1982.tb22125.x. [DOI] [PubMed] [Google Scholar]
- De Beer F. C., Pepys M. B. Isolation of human C-reactive protein and serum amyloid P component. J Immunol Methods. 1982;50(1):17–31. doi: 10.1016/0022-1759(82)90300-3. [DOI] [PubMed] [Google Scholar]
- Dyck R. F., Evans D. J., Lockwood C. M., Rees A. J., Turner D., Pepys M. B. Amyloid P-component in human glomerular basement membrane. Abnormal patterns of immunofluorescent staining in glomerular disease. Lancet. 1980 Sep 20;2(8195 Pt 1):606–609. doi: 10.1016/s0140-6736(80)90281-0. [DOI] [PubMed] [Google Scholar]
- Glenner G. G. Amyloid deposits and amyloidosis. The beta-fibrilloses (first of two parts). N Engl J Med. 1980 Jun 5;302(23):1283–1292. doi: 10.1056/NEJM198006053022305. [DOI] [PubMed] [Google Scholar]
- Glenner G. G. Amyloid deposits and amyloidosis: the beta-fibrilloses (second of two parts). N Engl J Med. 1980 Jun 12;302(24):1333–1343. doi: 10.1056/NEJM198006123022403. [DOI] [PubMed] [Google Scholar]
- Hind C. R., Collins P. M., Caspi D., Baltz M. L., Pepys M. B. Specific chemical dissociation of fibrillar and non-fibrillar components of amyloid deposits. Lancet. 1984 Aug 18;2(8399):376–378. doi: 10.1016/s0140-6736(84)90544-0. [DOI] [PubMed] [Google Scholar]
- Hind C. R., Collins P. M., Renn D., Cook R. B., Caspi D., Baltz M. L., Pepys M. B. Binding specificity of serum amyloid P component for the pyruvate acetal of galactose. J Exp Med. 1984 Apr 1;159(4):1058–1069. doi: 10.1084/jem.159.4.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JANIGAN D. T. EXPERIMENTAL AMYLOIDOSIS: STUDIES WITH A MODIFIED CASEIN METHOD, CASEIN HYDROLYSATE AND GELATIN. Am J Pathol. 1965 Jul;47:159–171. [PMC free article] [PubMed] [Google Scholar]
- Pepys M. B., Baltz M. L. Acute phase proteins with special reference to C-reactive protein and related proteins (pentaxins) and serum amyloid A protein. Adv Immunol. 1983;34:141–212. doi: 10.1016/s0065-2776(08)60379-x. [DOI] [PubMed] [Google Scholar]
- Pepys M. B., Baltz M. L., de Beer F. C., Dyck R. F., Holford S., Breathnach S. M., Black M. M., Tribe C. R., Evans D. J., Feinstein A. Biology of serum amyloid P component. Ann N Y Acad Sci. 1982;389:286–298. doi: 10.1111/j.1749-6632.1982.tb22144.x. [DOI] [PubMed] [Google Scholar]
- Pepys M. B., Dyck R. F., de Beer F. C., Skinner M., Cohen A. S. Binding of serum amyloid P-component (SAP) by amyloid fibrils. Clin Exp Immunol. 1979 Nov;38(2):284–293. [PMC free article] [PubMed] [Google Scholar]
- Pepys M. B. Isolation of serum amyloid P-component (protein SAP) in the mouse. Immunology. 1979 Jul;37(3):637–641. [PMC free article] [PubMed] [Google Scholar]
- Rowe I. F., Jensson O., Lewis P. D., Candy J., Tennent G. A., Pepys M. B. Immunohistochemical demonstration of amyloid P component in cerebro-vascular amyloidosis. Neuropathol Appl Neurobiol. 1984 Jan-Feb;10(1):53–61. doi: 10.1111/j.1365-2990.1984.tb00340.x. [DOI] [PubMed] [Google Scholar]
- Salacinski P. R., McLean C., Sykes J. E., Clement-Jones V. V., Lowry P. J. Iodination of proteins, glycoproteins, and peptides using a solid-phase oxidizing agent, 1,3,4,6-tetrachloro-3 alpha,6 alpha-diphenyl glycoluril (Iodogen). Anal Biochem. 1981 Oct;117(1):136–146. doi: 10.1016/0003-2697(81)90703-x. [DOI] [PubMed] [Google Scholar]
- Westermark P., Shirahama T., Skinner M., Brun A., Cameron R., Cohen A. S. Immunohistochemical evidence for the lack of amyloid P component in some intracerebral amyloids. Lab Invest. 1982 May;46(5):457–460. [PubMed] [Google Scholar]






