Abstract
Besides abundant oleosin, three minor proteins, Sop 1, 2, and 3, are present in sesame (Sesamum indicum) oil bodies. The gene encoding Sop1, named caleosin for its calcium-binding capacity, has recently been cloned. In this study, Sop2 gene was obtained by immunoscreening, and it was subsequently confirmed by amino acid partial sequencing and immunological recognition of its overexpressed protein in Escherichia coli. Immunological cross recognition implies that Sop2 exists in seed oil bodies of diverse species. Along with oleosin and caleosin genes, Sop2 gene was transcribed in maturing seeds where oil bodies are actively assembled. Sequence analysis reveals that Sop2, tentatively named steroleosin, possesses a hydrophobic anchoring segment preceding a soluble domain homologous to sterol-binding dehydrogenases/reductases involved in signal transduction in diverse organisms. Three-dimensional structure of the soluble domain was predicted via homology modeling. The structure forms a seven-stranded parallel β-sheet with the active site, S-(12X)-Y-(3X)-K, between an NADPH and a sterol-binding subdomain. Sterol-coupling dehydrogenase activity was demonstrated in the overexpressed soluble domain of steroleosin as well as in purified oil bodies. Southern hybridization suggests that one steroleosin gene and certain homologous genes may be present in the sesame genome. Comparably, eight hypothetical steroleosin-like proteins are present in the Arabidopsis genome with a conserved NADPH-binding subdomain, but a divergent sterol-binding subdomain. It is indicated that steroleosin-like proteins may represent a class of dehydrogenases/reductases that are involved in plant signal transduction regulated by various sterols.
Vegetable cooking oils are triacylglycerols (TAGs) extracted from various plant seeds. The storage of TAGs is confined to the discrete spherical organelles called oil bodies (Yatsu and Jacks, 1972; Murphy, 1993; Huang, 1996). The surface of an oil body appears to be entirely covered by proteins such that the compressed oil bodies in the cells of a mature seed never coalesce or aggregate (Slack et al., 1980; Tzen and Huang, 1992). This stability is attributed to the steric hindrance and electronegative repulsion of proteins, mostly structural proteins termed oleosins, on the surface of oil bodies (Tzen et al., 1992).
An oil body, 0.5 to 2.5 μm in diameter (Tzen et al., 1993), consists of a TAG matrix surrounded by a monolayer of phospholipids (PLs) embedded with abundant oleosins and some minor proteins (Tzen et al., 1997). Oleosins are alkaline proteins with a molecular mass of 15 to 25 kD depending on the species (Qu et al., 1986), and have been extensively investigated in the past decade (Napier et al., 1996; Frandsen et al., 2001). An oleosin molecule is proposed to be comprise of three distinct structural domains: an N-terminal domain, a central hydrophobic anchoring domain, and a C-terminal amphipathic α-helical domain (Vance and Huang, 1987). Sequence comparison among diverse species reveals that the central anchoring domain of oleosin is highly conserved, particularly in a relatively hydrophilic motif termed the Pro knot (Tzen et al., 1992). It is proposed that the Pro knot motif may play a crucial role in oleosin and caleosin targeting to oil bodies (Abell et al., 1997; Chen and Tzen, 2001).
Three minor proteins, temporarily termed Sop 1, 2, and 3, have been identified exclusively present in sesame (Sesamum indicum) oil bodies (Chen et al., 1998). However, the biological functions of these three minor proteins remain unknown. A cDNA sequence encoding sesame Sop1, named caleosin for its calcium-binding capacity, was recently cloned (Chen et al., 1999). Similar to oleosin in structure, caleosin is comprised of three distinct structural domains: an N-terminal hydrophilic domain (including a calcium-binding motif), a central hydrophobic anchoring domain, and a C-terminal hydrophilic domain. In addition, a comparable Pro knot motif is located in the central hydrophobic domain of caleosin. Whether the Pro knot motif is ubiquitously present in all oil body-associated proteins as an essential structural requirement remains to be investigated.
In animals and yeast, it has been well documented that steroids are membrane components and may also participate in signal transduction. Based on mutant studies, brassinosteroid is proposed to engage in plant development probably via a similar signal transduction pathway (Hartmann, 1998). The reported mutants lead to the identification of many genes encoding enzymes involved in the biosynthetic pathway of brassinosteroid (Schmacher and Chory, 2000). A membrane-bound brassinosteroid receptor has been lately identified and has proved to be a kinase that may transduce steroid signals across the plasma membrane (Wang et al., 2001). Recent studies on two Arabidopsis mutants suggest that some sterols other than brassinosteroid may also participate in signal transduction during plant development (Willmann, 2000). However, the mechanism and pathway of signal transduction via sterols in plant remain speculative. To date, there are no reports describing definite biological function or physiological regulation controlled by sterol signal transduction in plant systems.
In this study, we cloned a cDNA sequence and its corresponding genomic sequence encoding one of the unique oil-body proteins, Sop2, from maturing sesame seeds. The deduced protein, tentatively named steroleosin, seems to exist in diverse seed oil bodies, and comprises an oil body-anchoring segment preceding a sterol-binding dehydrogenase. Southern hybridization implies that one steroleosin gene and certain steroleosin-like genes may exist in the sesame genome. The results suggest that different sterol-binding dehydrogenases/reductases may be present in diverse plant tissues and may be involved in signal transduction.
RESULTS
Cloning of a Potential Gene Encoding Sop2, a Unique Protein in Oil Bodies of Sesame Seed
An incomplete cDNA clone presumably encoding sesame Sop2 was obtained by immunoscreening, and the upstream sequence of the clone was completed by PCR. The full-length cDNA clone (accession no. AF302806) was linked by ligation of the two overlapping fragments. The cDNA fragment comprises 1,357 nucleotides consisting of a 44-nucleotide 5′-untranslated region, an open reading frame of 1,047 nucleotides, and a 266-nucleotide 3′-untranslated region. The corresponding genomic sequence (2,440 nucleotides) of this putative Sop2 gene was also obtained by PCR cloning (accession no. AF421889). The open reading frame encodes a putative sterol-binding enzyme that belongs to the short chain dehydrogenase/reductase family (Duax and Ghosh, 1998). This encoded protein and its gene have not been reported in any species, except for the deduced polypeptide of a hypothetical mRNA theoretically spliced from Arabidopsis genome (Fig. 1). Sesame and Arabidopsis genomic sequences comprise six exons with five introns conservatively inserted in their coding regions. The deduced polypeptide of the sesame clone comprises 348 amino acid residues of Mr 39,570 D, close to the molecular mass of sesame Sop2 observed in SDS-PAGE (Fig. 2).
Figure 1.
Sequence alignment of sesame and Arabidopsis Sop2 sequences. The sequences are aligned according to four proposed structural regions (oil body-anchoring segment, NADPH binding subdomain, active site, and sterol-binding subdomain) of Sop2. The amino acid number for the last residue in each row is listed on the right for each species. A gap represented by a broken line is introduced between residues 241 (Ala) and 242 (Gly) of sesame Sop2 for best alignment. Three partial sequences obtained directly from amino acid sequencing are boxes. The three consensus residues in the active site are highlighted. Predicted secondary structures are indicated on the tops of the sequences (see Fig. 5B for details). The locations of α-helices and β-strands in the predicted Sop2 structure are indicated and are labeled successively. Locations where introns in their corresponding genomic sequences occur are indicated by triangles on tops of the sequences. The accession number of the aligned Arabidopsis Sop2 is BAA96983.
Figure 2.
SDS-PAGE and western blotting of the overexpressed sesame Sop2 in E. coli. Along with sesame oil body proteins and purified Sop2, the recombinant Sop2 (without oil body-anchoring segment) overexpressed in E. coli using a His-tag fusion vector was resolved in a 12.5% (w/v) SDS-PAGE gel. A duplicate gel was transferred onto nitrocellulose membrane and was then subjected to immunodetection using antibodies (1:1,500 dilution) against the seed-purified Sop2 protein. Labels on the left indicate the molecular masses of proteins.
Confirmation of Sop2 Clone by Amino Acid Sequencing and Immunological Recognition of Overexpressed Sop2 in E. coli
Three partial amino acid sequences, MDLIHTFLNLIA, MSFYNASKAAI, and YNAGERVIDQDM, were separately obtained from the intact polypeptide, a trypsin-digested fragment, and a chymotrypsin-digested fragment of Sop2 protein purified from sesame seed oil bodies. These three partial sequences are found in the deduced polypeptide of the obtained clone under the correct location or protease cleavage site (Fig. 1), and thus confirm the identity of sesame Sop2 clone. Furthermore, the cDNA sequence encoding the putative sterol-binding dehydrogenase/reductase domain (42–348 amino acid residues) in Sop2 clone was constructed in a His-tag fusion vector and was then overexpressed in E. coli. The overexpressed polypeptide was predominantly present in the insoluble pellet of E. coli lysate (Fig. 2). The insoluble pellet containing the overexpressed polypeptide was solubilized by urea, renatured by dialysis, purified by TALON resin, and then subjected to SDS-PAGE and immunodetection using antibodies raised against Sop2 purified from sesame seed oil bodies. The expressed recombinant polypeptide and the seed-purified Sop2 were equivalently recognized in the immunodetection. The results confirm again that the current clone encodes sesame Sop2, and they reveal that no removable signal sequence exists in Sop2 protein.
Immunological Cross Recognition of Sop2 in Oil Bodies of Various Oily Seeds
Proteins extracted from oil bodies of sesame and three other oily seeds (soybean [Glycine max], sunflower [Helianthus annus], and rapeseed [Brassica campestris]) were resolved in SDS-PAGE and subjected to immunodetection using antibodies against sesame Sop2 (Fig. 3). Polypeptides of molecular masses close to sesame Sop2 were cross-recognized in the three examined species. Putatively, Sop2 exists in seed oil bodies of diverse species.
Figure 3.
SDS-PAGE and western blotting of proteins extracted from seed oil bodies of various species. Proteins extracted from oil bodies of sesame and three other oily seeds were resolved in a 10% (w/v) SDS-PAGE gel. A duplicate gel was transferred onto nitrocellulose membrane and was then subjected to immunoassaying using antibodies (1:100 dilution) against sesame Sop2. Labels on the left indicate the molecular masses of proteins.
Concurrent Expression of Oleosin, Caleosin, and Sop2 Genes in Maturing Sesame Seeds
Accumulation of Sop2 mRNA appeared in maturing seeds approximately 2 weeks after flowering, and this mRNA maintained a substantial level thereafter until the late stage of seed maturation in a mode similar to oleosin or caleosin mRNA (Fig. 4). The result reveals that the Sop2 gene is transcribed along with oleosin and caleosin genes during seed maturation when oil bodies are actively assembled. This observation is in accordance with the exclusive accumulation of oleosin, caleosin, and Sop2 in oil bodies of maturing sesame seeds detected by western blots (Chen et al., 1998).
Figure 4.
Northern-blot analysis of total RNA extracted from various stages of maturing sesame seeds. Each lane was loaded with 20 μg of total RNA extracted from maturing seeds at various days after flowering (DAF). After blotting, the membrane was hybridized with a 32P-labeled probe containing the coding sequence of sesame Sop2, caleosin, or oleosin. Only the portion of the membrane corresponding to the visible hybridized RNA is shown.
Sequence Comparison among Various Sterol-Binding Dehydrogenases/Reductases of Diverse Organisms and Prediction of Sop2 Protein Structure
The sesame Sop2 is partially homologous to a sterol-binding dehydrogenase/reductase family found in diverse organisms. The sterol-binding dehydrogenases/reductases in this family may be membrane associated or located in the cytoplasm, depending on whether a transmembrane domain is present (Duax et al., 2000). Sequence alignment of sesame Sop2 with various sterol-binding dehydrogenases/reductases reveals that an extra N-terminal segment of approximately 40 amino acid residues is present in Sop2 (Fig. 5). Hydropathy plot indicates that the extra N-terminal segment in Sop2 is hydrophobic (Fig. 6A) and is presumably responsible for association with oil body membranes. In a similar manner, two of the aligned sequences, Human-1 and Droso-1, which also possess an extra N-terminal segment preceding the dehydrogenase/reductase core structure, may be membrane associated, whereas the rest of the aligned sequences that comprise merely the soluble dehydrogenase/reductase domain are probably located in the cytosol.
Figure 5.
Sequence alignment of sesame Sop2 with six sterol-binding dehydrogenase/reductase sequences. Sesame Sop2 is compared with different sterol-binding dehydrogenases/reductases of diverse species. The amino acid number for the last residue in each row is listed on the right for each species. Broken lines in the sequences represent gaps introduced for best alignment and conserved residues are shaded. The proposed structural regions (membrane anchoring, NADPH binding, and sterol binding) are indicated on the tops of the sequences. The three consensus residues in dehydrogenase/reductase active site are highlighted. The accession numbers of the aligned sequences are: Human-1 (Homo sapiens), AAC31757; Droso-1 (Drosophila melanogaster), AAF56927; Human-2, AAF06941; Droso-2, AAF45573; Strepto (Streptomyces clavuligerus), AAF86624; Bacillus (Bacillus subtilis), CAB14310.
Figure 6.
A, Hydropathy plot of sesame Sop2. The hydrophobicity scale was plotted versus amino acid sequence of sesame Sop2 with a window size of 19 using hydropathy index described by Kyte and Doolittle (1982). B, A secondary structural model of sesame steroleosin on the surface of an oil body. A monolayer of PLs, depicted by pink balls attached with two tails, segregates the hydrophobic TAG matrix (gradient yellow) of an oil body from hydrophilic cytosol (gradient light blue). Amino acid residues are represented by one-letter symbols in green circles. Numbers next to residues or secondary structures represent their relative positions counting from N terminus. Two structural domains are predicted in a steroleosin molecule: an N-terminal oil body-anchoring domain and a sterol-binding dehydrogenase domain. The hydrophobic N-terminal domain (residues 1–40) is supposed to associate with the monolayer PL of oil body surface by forming two amphipathic α-helices connected by a hydrophobic segment termed the Pro knob. The core structure of sterol-binding dehydrogenase domain forms a seven-stranded β-sheet surrounded by α-helices and can be divided into three regions: an NADPH-binding subdomain, an active site, and a sterol-binding subdomain. The three conserved residues (S, Y, and K) in the active site are indicated. NADPH and sterol are denoted by brown and orange molecules, respectively. C, Three-dimensional structural modeling of the sterol-binding dehydrogenase domain of sesame steroleosin. The three-dimensional structure of the soluble domain comprising the core structure of sterol-binding dehydrogenase was predicted using homology modeling (Lund et al., 1997).
It is proposed that Sop2, tentatively named steroleosin, possesses an N-terminal hydrophobic segment that anchors a soluble sterol-binding dehydrogenase/reductase on the surface of seed oil bodies (Fig. 6B). Sequence analysis of sesame and Arabidopsis steroleosin sequences (Fig. 1) suggests that the N-terminal anchoring segment is comprised of two amphipathic α-helices (12 residues in each helix) connected by a hydrophobic sequence of 14 residues bordered by 1–2 Pro at each end and rich in Phe and Leu residues. The two amphipathic α-helices are mostly composed of hydrophobic residues (hydrophobic/hydrophilic = 9/3) and thus are mainly embedded in the acyl portion of the PL monolayer. The relatively hydrophilic Pro residues located in both ends of the 14-residue hydrophobic sequence may aggregate in the hydrophobic surroundings and form a unique structure, tentatively termed the Pro knob motif, for the integrity and stability of steroleosin anchorage on the surface of oil bodies.
The soluble sterol-binding dehydrogenase/reductase domain of steroleosin can be divided into an NADPH-binding subdomain, an active site region, and a sterol-binding subdomain (Figs. 1 and 6B). Among different sterol-binding dehydrogenases/reductases, the NADPH-binding subdomain and the active site region, S-(12X)-Y-(3X)-K, are conserved, whereas the sterol-binding subdomain varies significantly in length and sequence (Fig. 5). The divergence of the sterol-binding subdomain among different sterol-binding dehydrogenases/reductases may be the result of the diversity of their binding sterols. The three-dimensional structure of the sterol-binding dehydrogenase/reductase domain of sesame steroleosin was predicted using comparative homology modeling (Fig. 6C). The core structure is composed of a seven-stranded parallel β-sheet sandwiched by α-helices. Compared with the cocrystal structure of the human 17 β-hydroxysteroid dehydrogenase with estradiol and NADP+ (Breton et al., 1996), the NADPH-binding region, active site, and sterol-binding region of steroleosin are putatively located in the C-terminal ends of the parallel β-strands (Fig. 6B). The NADPH-binding region is presumably located in the crevice region, termed the topological switch point, composed of loops between β-strands 1 and 4 as observed in all the similar α/β structures (Branden, 1980).
Detection of Sterol-Coupling Dehydrogenase Activity in Sesame Steroleosin
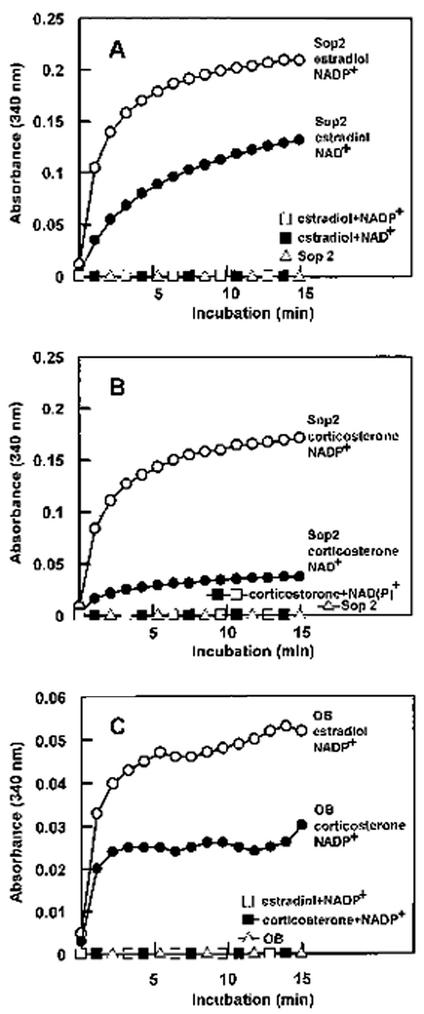
Based on the homology of sesame steroleosin to 17 β-hydroxysteroid (estradiol) dehydrogenase and 11 β-hydroxysteroid (corticosterone) dehydrogenase, estradiol and corticosterone were used to examine dehydrogenase activity of the overexpressed soluble domain of sesame steroleosin in the presence of coenzyme, NADP+, or NAD+. The results indicate that the soluble domain of steroleosin exerts dehydrogenase activity to both sterol substrates in the presence of either coenzyme (Fig. 7, A and B). In agreement with the homologous enzymes, steroleosin possesses higher dehydrogenase activity in the presence of NADP+ than in the presence of NAD+ regardless of the sterol substrates. Similar dehydrogenase activities were also detected using purified sesame oil bodies instead of the overexpressed soluble domain of steroleosin (Fig. 7C). In contrast, no reductase activity was detected in our experiments using estrane as a sterol substrate in the presence of NADPH (data not shown). The results suggest that steroleosin is an NADP+-binding sterol dehydrogenase with its endogenous sterol substrate possibly similar to hydroxysteroid.
Figure 7.
Spectrophotometric detection of dehydrogenase activity in sesame steroleosin (Sop2). The dehydrogenase activity of the expressed Sop2 soluble domain (25 μg) was detected using estradiol (A) or corticosterone (B) as a sterol substrate in the presence of NADP+ or NAD+. C, Sesame oil bodies (OB) containing 5 μg of Sop2 protein was assayed for dehydrogenase activity using the same sterol substrates in the presence of NADP+.
Southern Analysis of Potential Steroleosin Homologous Genes in Sesame Genome
To detect the copy number of the steroleosin gene and to examine potential steroleosin homologous genes in the sesame genome, a cDNA fragment encoding the oil body-anchoring domain and part of the NADPH-binding subdomain was 32P labeled as a probe to hybridize genomic DNA digested with three restriction enzymes (Fig. 8). One major and several minor fragments were detected in the three conditions of enzymatic digestion. It is assumed that one steroleosin gene and several steroleosin-like genes may be present in the sesame genome.
Figure 8.
Southern-blot analysis of genomic DNA extracted from leaves of sesame plants. Each lane was loaded with 10 μg of genomic DNA completely digested with EcoRI, HindIII, or PstI. After blotting, the membrane was hybridized with a 32P-labeled probe containing part of the coding sequence of sesame steroleosin.
DISCUSSION
Sop2 is a minor protein of seed oil bodies first identified in sesame (Chen et al., 1998). In this study, the corresponding cDNA sequence and genomic sequence of Sop2 were obtained, and the deduced polypeptide was named steroleosin for its homology to a sterol-binding dehydrogenase/reductase class involved in signal transduction in diverse organisms. Steroleosin seems to exist in seed oil bodies of diverse species. Similar to oleosin and caleosin, steroleosin is a unique oil body protein expressed in developing seeds. Although seed oleosin and oleosin-like proteins encoded by its homologous genes are assumed to exist exclusively in oil bodies, several homologous genes encoding caleosin-like proteins are found expressed in various tissues including non-oil storage tissues (Næsted et al., 2000). Based on this study, putative homologous genes encoding steroleosin-like proteins that are unlikely associated with oil bodies are possibly expressed in various non-oil storage tissues in a manner similar to caleosin-like but not oleosin-like genes.
A cleavable signal sequence and post-translational modification are not present in steroleosin purified from sesame seed oil bodies, just as in oleosin and caleosin (Chen et al., 1997, 1999), whereas the N-terminal amino group of oleosin or caleosin (but not steroleosin) is blocked when subjected to amino acid sequencing using the intact proteins eluted from SDS-PAGE gels. The reason for the discrepancy of N-terminal blocking in these three oil body proteins is unclear. In contrast with oleosin and caleosin, whose hydrophobic anchoring domains are located in the central portions of their protein structures, steroleosin anchors a soluble dehydrogenase on the surface of oil bodies via its N-terminal hydrophobic segment. The unique Pro knot motif that occurs in the middle of the central hydrophobic domain of oleosin or caleosin is not present in steroleosin. Instead, a Pro knob motif is found in the middle of the N-terminal hydrophobic segment of steroleosin. Pro knot and Pro knob motifs contain three to four Pro residues in a very hydrophobic sequence but with different structural organization. Meanwhile, the Pro knot motif in oleosin and caleosin, but not the Pro knob motif in steroleosin, is connected with paired hydrophobic antiparallel β-strands. Whether the Pro knob motif in steroleosin is equivalent to the Pro knot motif in oleosin or caleosin and crucial for steroleosin targeting to oil bodies remains to be elucidated.
In agreement with the proposed steroleosin gene family in sesame, eight steroleosin homologous genes are present in the Arabidopsis genome. Moreover, potential regulatory elements specifically responsive in developing flower, leaves, or immature fiber are found in the putative promoter regions of several steroleosin homologous genes in Arabidopsis. All the eight hypothetical steroleosin-like proteins possess an N-terminal appendix preceding a sterol-binding dehydrogenase/reductase domain (Fig. 9). Sequence analysis of the N-terminal appendices suggests that Arab-1 and Arab-2 are oil body associated; Arab-3 and Arab-4 are membrane associated; Arab-5, Arab-6, and Arab-7 may or may not be membrane bound; and Arab-8 is water soluble and presumably present in cytosol. Among these Arabidopsis steroleosin-like proteins, the NADPH-binding subdomain and the active site region are conserved, whereas the sterol-binding subdomain varies significantly in length and sequence. According to intron organization, these steroleosin homologous genes contain five conserved intron locations, except the Arab-8 gene, which comprises 11 introns. Meanwhile, the Arab-1 gene possesses two copies (BAB09145 and BAA96983) that are tightly associated with, but in the opposite direction of, the Arab-3 gene (BAB09144) and the Arab-4 gene (BAA96982), respectively. Arab-1, -3, and -4 genes, together with the Arab-2 gene (BAA96990), are closely located in chromosome V of the Arabidopsis genome.
Figure 9.
Sequence alignment of eight putative sterol-binding dehydrogenase/reductase sequences in Arabidopsis. The amino acid number for the last residue in each row is listed on the right for each species. Broken lines in the sequences represent gaps introduced for best alignment and conserved residues are shaded. The proposed structural regions (N-terminal appendix, NADPH binding, and sterol binding) are indicated on the tops of the sequences. The three consensus residues in the dehydrogenase/reductase active site are highlighted. Locations where introns in their corresponding genomic sequences occur are indicated by triangles on the tops of the sequences. The accession numbers of Arab-1 through -8 sequences are: BAA96983, BAA96990, BAB09144, BAA96982, CAB51207, CAB51208, CAB39626, and AAF01606.
Sterol-binding dehydrogenases/reductases comprise a superfamily involved in diverse signal transduction (Duax et al., 2000). It is proposed that steroleosin may be involved in signal transduction regulating a specialized biological function related to seed oil bodies. The putative biological function may be affiliated to the mobilization of oil bodies during seed germination. Brassinosteroid and other unknown sterols have recently been suggested to play important roles during plant development, though detailed mechanisms and regulatory pathways have not been identified (Schmacher and Chory, 2000). Advanced investigation in this research field has been impeded, as no representative system of definite biological function or physiological regulation is available at this moment. The findings of the current study, i.e. identification of steroleosin in seed oil body and implication of steroleosin-like proteins in non-oil storage tissues, provide a working system to study the pathways of signal transduction in plant sterols. The observed diversity of sterol-binding domain of different steroleosin-like proteins in the Arabidopsis genome (Fig. 9) implies that diverse sterols may bind to specific steroleosin-like proteins and may initiate signal transduction to drive various biological pathways in plant tissues.
In mammals and microorganisms, many a sterol-binding dehydrogenase/reductase has been identified as a presignal protein involved in signal transduction via activation of its partner receptor after binding to a regulatory sterol (Stewart and Krozowski, 1999). In some examples, sterol-binding dehydrogenase/reductase and its partner receptor are demonstrated to form a heterodimer on the cell membrane where an extracellular sterol hormone presumably targets during signal transduction. In seed oil bodies, the abundance of steroleosin is similar to that of caleosin (27 kD), another seed oil body protein of unknown function (Fig. 2). Moreover, caleosin is comprised of a calcium-binding motif and several potential phosphorylation sites that are well-known candidates involved in signal transduction. Whether steroleosin serves as a presignal molecule associated with caleosin as its partner receptor on seed oil bodies remains to be seen.
MATERIALS AND METHODS
Plant Materials
Mature and fresh maturing sesame (Sesamum indicum) seeds were gifts from the Crop Improvement Department (Tainan District Agricultural Improvement Station). Mature seeds of soybean (Glycine max), sunflower (Helianthus annus), and rapeseed (Brassica campestris) were purchased from local seed stores.
Antibody Preparation
Sesame Sop2 protein was eluted from SDS-PAGE gels according to the method described by Chuang et al. (1996). Antibodies against sesame Sop2 were raised in chickens and were purified from egg yolks (Polson, 1990). Preimmune eggs taken from the chickens 1 week before antigen injection were used as preimmune blotting controls. Two chickens whose preimmune antibodies did not recognize any sesame oil body proteins were selected for antigen injection. The antigen (Sop2 in a solution of 1 mg mL−1) was mixed with an equal volume of complete Freund's adjuvant. A volume of 1 mL of the antigen mixture was injected into the chest muscle of each chicken. Booster injections of equal amounts of the antigen were given 10 and 20 d after the first injection, except for the use of incomplete Freund's adjuvant instead of complete Freund's adjuvant. One week after the second booster injection, eggs were collected daily. Immunoglobulins were purified from the egg yolks, aliquoted, and stored at −80°C in the presence of 0.1% (w/v) sodium azide.
Isolation of Poly(A)+ RNA and cDNA Library Construction
Total RNA was extracted from the maturing seeds (24 d after flowering) ground in liquid nitrogen using the phenol/SDS method (Wilkins and Smart, 1996). Poly(A)+ RNA was isolated with Dynabeads (Dynal Biotech, Oslo) following the manufacturer's instructions. The isolated poly(A)+ RNA was dissolved in diethyl pyrocarbonate-treated water and was then quantitated as the A260 with a spectrophotometer. cDNA was synthesized from poly(A)+ RNA according to the protocol described in the manufacturer's instructions (cDNA synthesis, ZAP-cDNA synthesis, and ZAP-cDNA Gigapack III Gold Cloning kits purchased from Stratagene, La Jolla, CA). A cDNA library of approximately 106 plaques was constructed with 5 μg of poly(A)+ RNA.
Immunoscreening and Sequencing
The cDNA library was plated on NZY agar plates at a density of 500 clones 15 cm−1 plate. The plates were incubated at 42°C for 4 h to allow plaque development. Nitrocellulose filters soaked with isopropyl β-d-thiogalactoside were then laid on top of the plaques and were incubated at 37°C for 4 h to transfer the plaques onto the membranes. The filters were blocked with 3% (w/v) gelatin in Tris-buffered saline (TBS) containing 20 mm Tris-HCl, pH 7.5, and 2 mm NaCl for 3 h at room temperature. To screen the library, antibodies against sesame Sop2 were diluted 1:100 in TBS buffer supplemented with 1% (w/v) gelatin, and were incubated with the filters at room temperature overnight. After antibody probing, the filters were washed with 0.05% (w/v) Tween 20 in TBS buffer and were then incubated with secondary antibody, peroxidase-conjugated AffiniPure rabbit anti-chicken IgG (Jackson Immunoresearch Laboratories, West Grove, PA) at 1:3,000 dilution in TBS buffer supplemented with 1% (w/v) gelatin for 2 h at room temperature. The filters were subsequently washed with 0.05% (w/v) Tween 20 in TBS buffer and were then treated with 4-chloro-1-naphthol containing H2O2 for color development. The plaques exhibiting immunoreactivity were excised from the plates, and the phages were converted into the pBluescript phagemid by in vivo excision with Exassist helper phage following the manufacturer's instructions (Stratagene). The excised phagemids were purified and subjected to automated DNA sequencing with T3 forward and T7 reverse primers. The insert fragment of 1,085 bp was identified as an incomplete clone when compared with the homologous sequence predicted in Arabidopsis genome. The upstream sequence of the clone was obtained by PCR amplification using a 23-nulceotide primer designed according to a sequence of the obtained fragment and a primer corresponding to the T3 promoter in the phagemid vector. An upstream fragment of 486 bp was harvested, ligated into the pGEM-T Easy Vector systems (Promega, Madison, WI), and subjected to sequencing. Because a PstI restriction enzyme site is present in the overlapping region of the 1,085-bp fragment and the 486-bp fragment, the complete Sop2 clone of 1,357 bp was linked by ligation of the two fragments digested with PstI.
Genomic Cloning of Sesame Sop2 Gene by PCR Amplification
Genomic DNA was isolated from sesame leaves according to the protocol described by Sambrook et al. (1989). The corresponding genomic DNA of sesame Sop2 gene was amplified by PCR using a pair of primers (5′-ATGGATCTAATCCACACTTTCCTCAAC-3′ and 5′-TTAATCATTCTTGGGCTCCGGAACTTG-3′) according to the open reading fragment of the Sop2 cDNA sequence. A PCR fragment of 2,440 bp was harvested, ligated into the pGEM-T Easy Vector systems, and subjected to sequencing. The entire sequence of the clone was completed using several designed primers within the sequence of the clone.
Overexpression of the Sesame Sop2 Clone in Escherichia coli
The cDNA fragment encoding the sterol-binding dehydrogenase/reductase domain of sesame Sop2 was constructed in the fusion expression vector, pQE30b(+) (QIAGEN, Valencia, CA), using a BamHI and a HindIII site in the polylinker of the vector. The recombinant plasmid was used to transform E. coli strain NovaBlue. Overexpression was induced by 0.1 mm isopropyl β-d-thiogalactoside in a bacteriophage T7 RNA polymerase/promoter system. Three hours after induction, the E. coli cells were harvested and crashed by sonication in the extraction/washing buffer containing 300 mm NaCl and 50 mm NaH2PO4, pH 7.0.
Affinity Purification Using TALON Resin
The sonicate was clarified by centrifugation at 14,000 rpm at 4°C for 15 min. The pellet was resuspended in 7 m urea and was dialyzed against 10 mm sodium phosphate buffer, pH 7.5, for 4 h at 4°C. After dialysis, the sample was centrifuged at 14,000 rpm at 4°C for 5 min and the supernatant was incubated with TALON resin (CLONTECH, Palo Alto, CA) for 20 min at room temperature. The resin was then spun down at 700g and was washed with the extraction/washing buffer by gentle end-over-end mixing at 4°C for 10 min. The His-tagged protein was eluted with the imidazole elution buffer containing 150 mm imidazole in extraction/washing buffer.
Purification of Oil Bodies
Oil bodies were extracted from mature seeds of sesame, soybean, sunflower, and rapeseed, and were then subjected to further purification using the protocol developed by Tzen et al. (1997), including two-layer flotation by centrifugation, detergent washing, ionic elution, treatment of chaotropic agent, and integrity testing with hexane.
SDS-PAGE and Western Blotting
Proteins extracted from various samples were resolved by SDS-PAGE using 10% or 12.5% (w/v) polyacrylamide in the separating gel and 4.75% (w/v) polyacrylamide in the stacking gel (Laemmli, 1970). After electrophoresis, the gel was stained with Coomassie Blue R-250 and was destained. In the immunoassaying, proteins in an SDS-PAGE gel were transferred onto nitrocellulose membrane in a Trans-Blot system (Bio-Rad, Hercules, CA) according to the manufacturer's instructions. The membrane was subjected to immunodetection using Sop2 antibodies (1:1,500 dilution for recognition of purified Sop2 and expressed Sop2 or 1:100 dilution for cross recognition of homologous Sop2 proteins from other seed oil bodies) or preimmune antibodies as negative controls. After washing, the membrane was supplemented with secondary antibodies (1:3,000 dilution) conjugated with horseradish peroxidase, and was then incubated with 4-chloro-1-naphthol containing H2O2 for color development (Chen et al., 1998).
Partial Amino Acid Sequencing
Sop2 protein eluted from SDS-PAGE gels was subjected to trypsin or chymotrypsin digestion. In the reaction mixture, 20 μg of Sop2 was digested with 5 μg of trypsin (bovine pancreas type III) or chymotrypsin (bovine pancreas type II) at 37°C for 30 min in a buffer of 50 mm Tris-HCl, pH 7.5. After digestion, the reaction mixture was added to an equal volume of 2× SDS-PAGE sample buffer and was boiled for 5 min. The hydrolysis products as well as intact Sop2 protein were resolved in an SDS-PAGE gel using 15% and 4.75% (w/v) polyacrylamide in the separating gel and stacking gel, respectively. After electrophoresis, fragments of polypeptide were transferred onto a piece of polyvinylidene difluoride membrane at a current of 0.5 Amps for 30 min at 4°C in a blotting buffer of 10% (w/v) methanol and 10 mm CAPS [3-(cyclohexylamino)propanesulfonic acid]-NaOH, pH 11. After blotting, the polyvinylidene difluoride membrane was stained with Coomassie Blue for 5 min, destained for 5 min, rinsed with water three times, and then left to dry in the air. The major stained band in each digestion as well as intact Sop2 protein was picked up for sequencing from the N terminus using the 476A Protein Sequencer (Applied Biosystems, Foster City, CA) in Chung-Hsing University (Taiwan).
Assay of Dehydrogenase Activity
Dehydrogenase activity of the overexpressed Sop2 (containing the soluble enzyme domain without oil body-anchoring segment) and oil bodies purified from sesame seed was assayed at 37°C by spectrophotometric measurement of NADP+ or NAD+ reduction indicated by the absorbance increase at 340 nm (Pu and Yang, 2000). The reaction mixture contained 25 μg of Sop2 protein or 15 mg of oil bodies (containing 5 μg of Sop2 protein), 5 mm NADP+ or NAD+, and 125 μm estradiol (17 β-hydroxysteroid) or corticosterone (11 β-hydroxysteroid) in 10 mm sodium phosphate buffer, pH 7.5, containing 0.6 m Suc.
Isolation of RNA and Northern-Blot Analysis
Total RNA from various stages of maturing seeds was extracted in liquid nitrogen using the phenol/SDS method (Wilkins and Smart, 1996). The isolated RNA of 20 μg was resolved in a 1.5% (w/v) formaldehyde/agarose gel, transferred onto a Hybond-N nylon membrane (Amersham Biosciences, Piscataway, NJ), and fixed by UV irradiation. The membrane was hybridized at 65°C for 14 h using a 32P-labeled probe containing the coding sequence of sesame Sop2, oleosin, or caleosin. The membrane was washed four times at 65°C following the manufacturer's instructions and was exposed to x-ray film.
Southern-Blot Analysis
Isolated genomic DNA of 10 μg was digested with EcoRI, HindIII, or PstI at 37°C overnight, and the resulting fragments were resolved in a 0.8% (w/v) agarose gel, transferred onto a piece of blotting membrane (Sartorius, Göttingen, Germany), and fixed by UV irradiation. The membrane was hybridized at 65°C for 14 h using an [α-32P]dCTP-labeled PCR product corresponding to nucleotides +1 to +291 of sesame Sop2 coding sequence. The membrane was washed three times at 65°C following the manufacturer's instructions and was exposed to x-ray film.
Sequence Analyses
Sequence comparisons were performed with the GenBank using the Blast program (Altschul et al., 1990). The unique motif of the short chain dehydrogenase/reductase family was identified using Motif program at the GenomeNet, Japan (http://www.motif.genome.ad.jp). Amphipathic α-helix was predicted using helix wheel projection (Shiffer and Edmundson, 1967) and helical hydrophobic moment (Eisenberg et al., 1982). Hydropathy profile was plotted with a window size of 19 using hydropathy index described by Kyte and Doolittle (1982). Protein three-dimensional structure was predicted in the CPHmodels World Wide Web server (http://www.cbs. dtu.dk/services/CPHmodels/) using comparative homology modeling (Lund et al., 1997). The three-dimensional modeling structure was rendered using the RasWin Molecular Graphics program (version 2.72). Potential responsive elements in promoter regions are searched in the TFSEARCH program: Searching Transcription Factor Binding Sites (version 1.3; http://molsun1.cbrc.aist.go.jp/research/db/TFSEARCH.html).
ACKNOWLEDGMENTS
We thank Prof. Chih-Ning Sun for critical reading of the manuscript, Dr. Tien-Joung Yiu of the Crop Improvement Department, Tainan District Agricultural Improvement Station for supplying mature and fresh maturing sesame seeds, and Ms. Miki Wang for preparation of Sop2 antibodies.
Footnotes
This work was supported by the National Science Council, Taiwan, Republic of China (grant no. NSC 89–2313–B–005–095 to J.T.C.T.).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.010928.
LITERATURE CITED
- Abell BM, Holbrook LA, Abenes M, Murphy DJ, Hills MJ, Moloney MM. Role of the proline knot motif in oleosin endoplasmic reticulum topology and oil body targeting. Plant Cell. 1997;9:1481–1493. doi: 10.1105/tpc.9.8.1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altschul SF, Warren G, Webb M, Eugene WM, David JL. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Branden CI. Relation between structure and function of α/β proteins. Q Rev Biophys. 1980;13:317–338. doi: 10.1017/s0033583500001712. [DOI] [PubMed] [Google Scholar]
- Breton R, Housset D, Mazza C, Fontecilla-Camps JC. The structure of a complex of human 17β-hydroxysteroid dehydrogenase with estradiol and NADP+identifies two principal targets for the design of inhibitors. Structure. 1996;15:905–915. doi: 10.1016/s0969-2126(96)00098-6. [DOI] [PubMed] [Google Scholar]
- Chen ECF, Tai SSK, Peng CC, Tzen JTC. Identification of three novel unique proteins in seed oil bodies of sesame. Plant Cell Physiol. 1998;39:935–941. doi: 10.1093/oxfordjournals.pcp.a029457. [DOI] [PubMed] [Google Scholar]
- Chen JCF, Lin RH, Huang HC, Tzen JTC. Cloning, expression and isoform classification of a minor oleosin in sesame oil bodies. J Biochem. 1997;122:819–824. doi: 10.1093/oxfordjournals.jbchem.a021828. [DOI] [PubMed] [Google Scholar]
- Chen JCF, Tsai CCY, Tzen JTC. Cloning and secondary structure analysis of caleosin, a unique calcium-binding protein in oil bodies of plant seeds. Plant Cell Physiol. 1999;40:1079–1086. doi: 10.1093/oxfordjournals.pcp.a029490. [DOI] [PubMed] [Google Scholar]
- Chen JCF, Tzen JTC. An in vitro system to examine the effective phospholipids and structural domain for protein targeting to seed oil bodies. Plant Cell Physiol. 2001;42:1245–1252. doi: 10.1093/pcp/pce160. [DOI] [PubMed] [Google Scholar]
- Chuang RLC, Chen JCF, Chu J, Tzen JTC. Characterization of seed oil bodies and their surface oleosin isoforms from rice embryos. J Biochem. 1996;120:74–81. doi: 10.1093/oxfordjournals.jbchem.a021396. [DOI] [PubMed] [Google Scholar]
- Duax WL, Ghosh D. Structure and mechanism of action and inhibition of steroid dehydrogenase enzymes involved in hypertension. Endocr Res. 1998;24:521–529. doi: 10.3109/07435809809032641. [DOI] [PubMed] [Google Scholar]
- Duax WL, Ghosh D, Pletnev V. Steroid dehydrogenase structures, mechanism of action, and disease. Vitam Horm. 2000;58:121–148. doi: 10.1016/s0083-6729(00)58023-6. [DOI] [PubMed] [Google Scholar]
- Eisenberg D, Weiss RM, Terwilliger TC. The helical hydrophobic moment: a measure of the amphilicity of a helix. Nature. 1982;299:371–374. doi: 10.1038/299371a0. [DOI] [PubMed] [Google Scholar]
- Frandsen GI, Mundy J, Tzen JTC. Oil bodies and their associated proteins, oleosin and caleosin. Physiol Plant. 2001;112:301–307. doi: 10.1034/j.1399-3054.2001.1120301.x. [DOI] [PubMed] [Google Scholar]
- Hartmann MA. Plant sterols and the membrane environment. Trends Plant Sci. 1998;3:170–175. [Google Scholar]
- Huang AHC. Oleosin and oil bodies in seeds and other organs. Plant Physiol. 1996;110:1055–1061. doi: 10.1104/pp.110.4.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyte J, Doolittle RF. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982;157:105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lund O, Frimand K, Gorodkin J, Bohr H, Bohr J, Hansen J, Brunak S. Protein distance constraints predicted by neural networks and probability density functions. Prot Eng. 1997;10:1241–1248. doi: 10.1093/protein/10.11.1241. [DOI] [PubMed] [Google Scholar]
- Murphy DJ. Structure, function and biogenesis of storage lipid bodies and oleosins in plants. Prog Lipid Res. 1993;32:247–280. doi: 10.1016/0163-7827(93)90009-l. [DOI] [PubMed] [Google Scholar]
- Napier JA, Stobart AK, Shewry PR. The structure and biogenesis of plant oil bodies: the role of the ER membrane and the oleosin class of proteins. Plant Mol Biol. 1996;31:945–956. doi: 10.1007/BF00040714. [DOI] [PubMed] [Google Scholar]
- Næsted H, Frandsen GI, Jauh GY, Hernandez-Pinzon I, Nielsen HB, Murphy DJ, Rogers JC, Mundy J. Caleosins: Ca2+binding proteins associated with lipid bodies. Plant Mol Biol. 2000;44:463–476. doi: 10.1023/a:1026564411918. [DOI] [PubMed] [Google Scholar]
- Polson A. Isolation of IgY from the yolks of eggs by a chloroform polyethylene glycol procedure. Immunol Invest. 1990;19:253–258. doi: 10.3109/08820139009041840. [DOI] [PubMed] [Google Scholar]
- Pu X, Yang K. Guinea pig 11 β-hydroxysteroid dehydrogenase type 1: primary structure and catalytic properties. Steroids. 2000;65:148–156. doi: 10.1016/s0039-128x(99)00098-7. [DOI] [PubMed] [Google Scholar]
- Qu R, Wang SM, Lin YH, Vance VB, Huang AHC. Characteristics and biosynthesis of membrane proteins of lipid bodies in the scutella of maize (Zea maysL.) Biochem J. 1986;234:57–65. doi: 10.1042/bj2350057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: A Laboratory Manual. Ed 2. Cold Spring, New York: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- Schmacher K, Chory J. Brassinosteroid signal transduction: still casting the actors. Curr Opin Plant Biol. 2000;3:79–84. doi: 10.1016/s1369-5266(99)00038-2. [DOI] [PubMed] [Google Scholar]
- Shiffer M, Edmundson AB. Use of helical wheels to represent the structures of proteins and to identify segments with helical potential. Biophys J. 1967;7:121–135. doi: 10.1016/S0006-3495(67)86579-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slack CR, Bertaud WS, Shaw BP, Holl R, Browse J, Wright H. Some studies on the composition and surface properties of oil bodies from the seed cotyledons of safflower and linseed. Biochem J. 1980;190:551–561. doi: 10.1042/bj1900551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart PM, Krozowski ZS. 11 β-Hydroxysteroid dehydrogenase. Vitam Horm. 1999;57:249–232. [PubMed] [Google Scholar]
- Tzen JTC, Cao YZ, Laurent P, Ratnayake C, Huang AHC. Lipids, proteins, and structure of seed oil bodies from diverse species. Plant Physiol. 1993;101:267–276. doi: 10.1104/pp.101.1.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzen JTC, Huang AHC. Surface structure and properties of plant seed oil bodies. J Cell Biol. 1992;117:327–335. doi: 10.1083/jcb.117.2.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzen JTC, Lie GC, Huang AHC. Characterization of the charged components and their topology on the surface of plant seed oil bodies. J Biol Chem. 1992;267:15626–15634. [PubMed] [Google Scholar]
- Tzen JTC, Peng CC, Cheng DJ, Chen ECF, Chiu JMH. A new method for seed oil body purification and examination of oil body integrity following germination. J Biochem. 1997;121:762–768. doi: 10.1093/oxfordjournals.jbchem.a021651. [DOI] [PubMed] [Google Scholar]
- Vance VB, Huang AHC. The major protein from lipid bodies of maize: characterization and structure based on cDNA cloning. J Biol Chem. 1987;262:11275–11279. [PubMed] [Google Scholar]
- Wang ZY, Seto H, Fujioka S, Yoshida S, Chory J. BRI1 is a critical component of a plasma-membrane receptor for plant steroids. Nature. 2001;410:380–383. doi: 10.1038/35066597. [DOI] [PubMed] [Google Scholar]
- Wilkins TA, Smart LB. Isolation of RNA from plant tissue. In: Krieg PA, editor. A Laboratory Guide to RNA. New York: Wiley-Liss; 1996. pp. 21–41. [Google Scholar]
- Willmann MR. Sterols as regulators of plant embryogenesis. Trends Plant Sci. 2000;5:416. doi: 10.1016/s1360-1385(00)91717-5. [DOI] [PubMed] [Google Scholar]
- Yatsu LY, Jacks TJ. Spherosome membranes: half unit-membranes. Plant Physiol. 1972;49:937–943. doi: 10.1104/pp.49.6.937. [DOI] [PMC free article] [PubMed] [Google Scholar]