Abstract
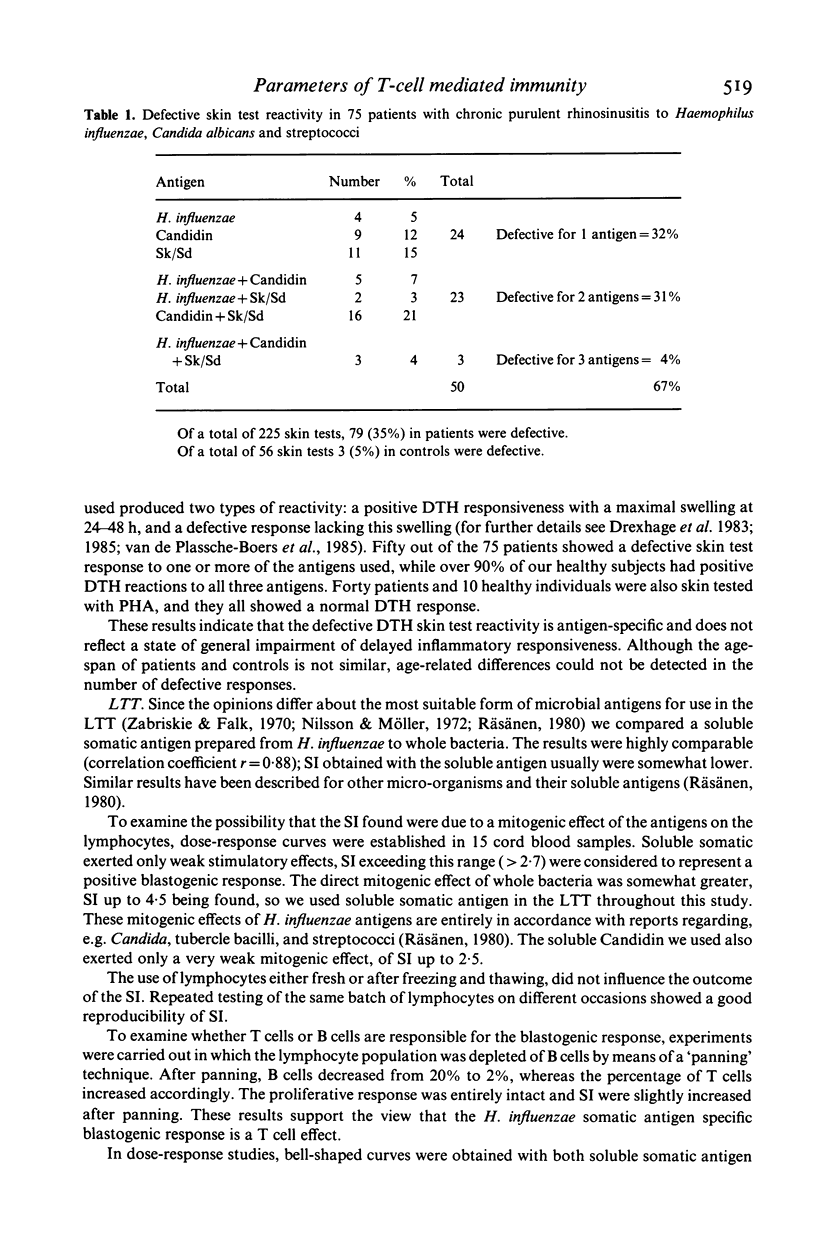
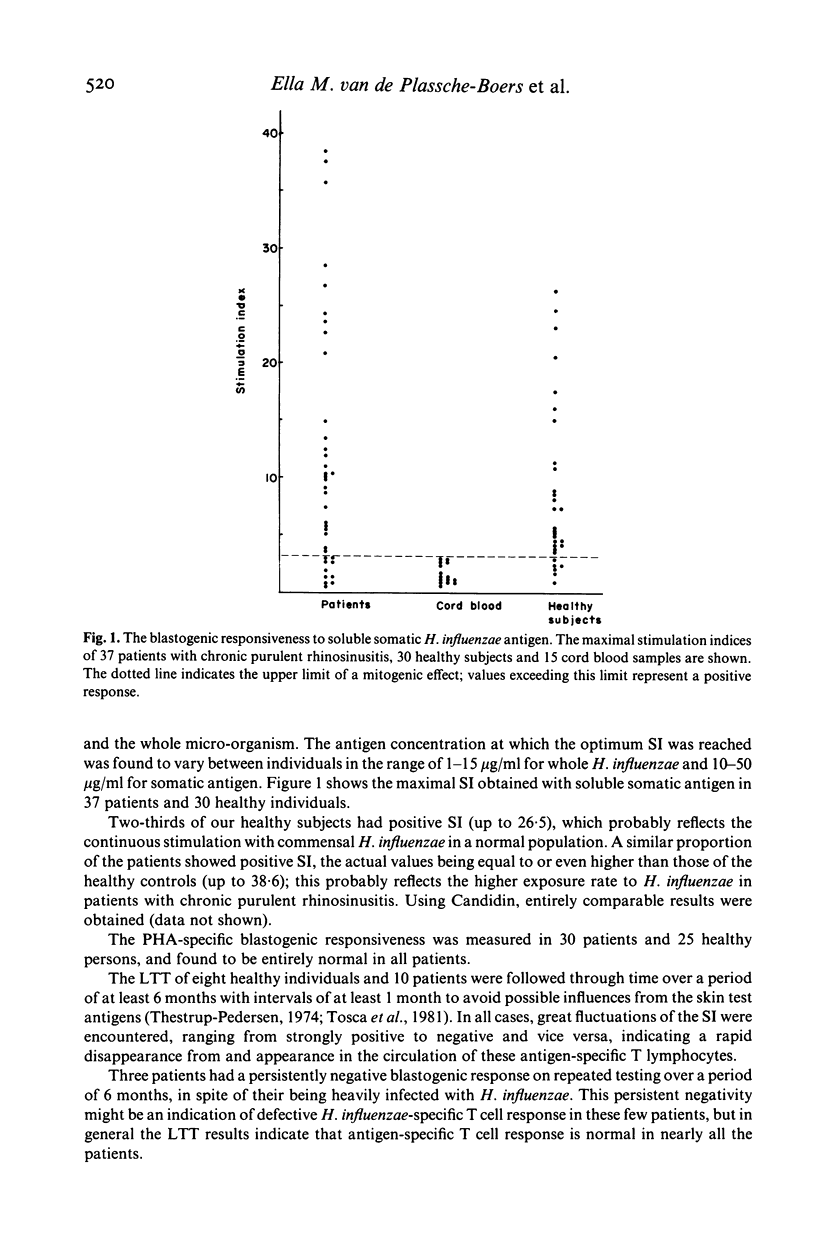
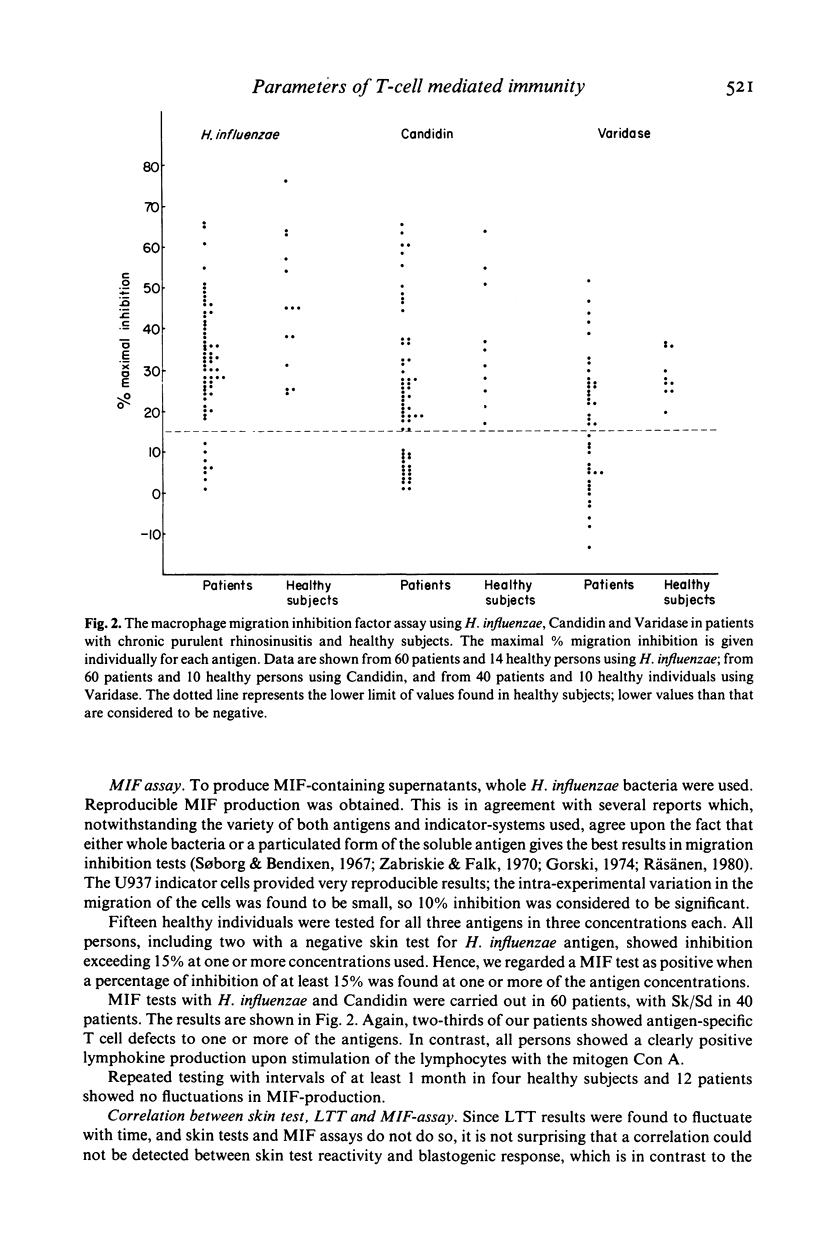
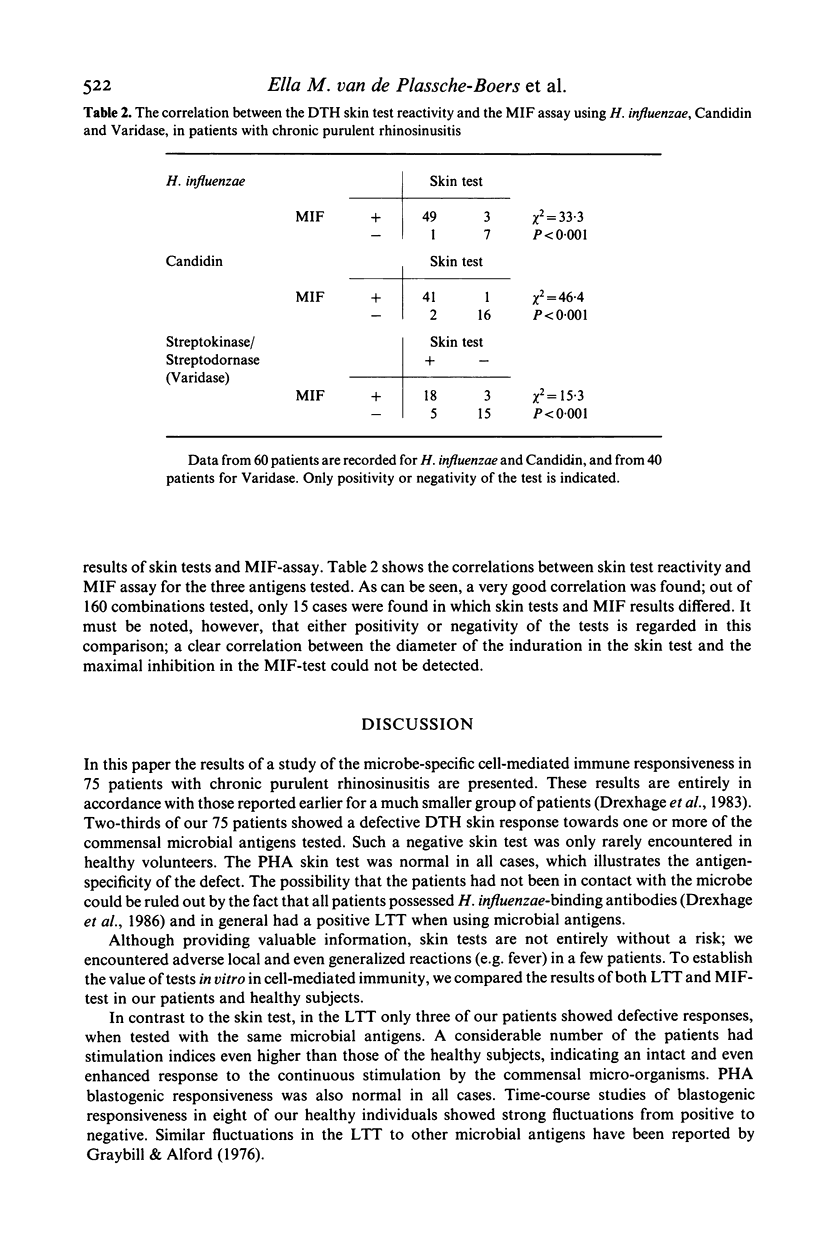
In 75 patients with unexplained chronic purulent rhinosinusitis T cell mediated immunity to three micro-organisms frequently colonizing the human upper respiratory tract, viz. Haemophilus influenzae, streptococci and Candida albicans, was assessed. Delayed type hypersensitivity (DTH) skin test reactivity was measured in vivo, whereas the blastogenic responsiveness (lymphocyte transformation test; LTT) and lymphokine production (e.g. migration inhibition factor; MIF) of the lymphocytes upon antigen stimulation were measured in vitro. MIF was assayed with a recently developed test system using the human monocytoid cell-line U937 as indicator cells in agarose microdroplets. Two-thirds of the 75 patients tested showed a defective DTH response to one or more of the microbial antigens; this contrasted to the findings in 25 healthy subjects, of whom over 90% showed a positive DTH reaction to any of the three antigens. PHA skin tests were entirely normal in both patients and healthy controls. Microbial antigen-specific LTT responses fluctuated considerably in time from strongly positive to negative and vice versa in healthy individuals as well as in patients. In general however, blastogenic responses in patients were comparable to or even higher than those of healthy persons. In the MIF assay, lymphocytes of all healthy individuals tested showed production of MIF upon stimulation with all three antigens; this again contrasted to two-thirds of the patients, whose lymphocytes showed a defective MIF production. Fluctuations of MIF-production in time could not be established and a very good correlation existed between the data obtained in the MIF assay and those of the DTH skin tests. These results indicate that apart from skin testing, the MIF assay seems to be the most suitable parameter to assess defects in T cell reactivity towards microbial antigens. These defects exist in two-thirds of our patients suffering from chronic purulent rhinosinusitis.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bernhard J. D., Rosenfeld S. S., Klein E. Blocking of delayed hypersensitivity by humoral antibody in an in vivo mouse transfer assay using sensitized guinea pig cells. Cell Immunol. 1973 Sep;8(3):408–412. doi: 10.1016/0008-8749(73)90131-7. [DOI] [PubMed] [Google Scholar]
- Clausen J. E. Migration inhibitory effect of cell-free supernatants from tuberculin-stimulated cultures of human mononuclear leukocytes demonstrated by two-step MIF agarose assay. J Immunol. 1973 Feb;110(2):546–551. [PubMed] [Google Scholar]
- Drexhage H. A., Oort J. Skin test reactivity to H. influenzae antigens as an outcome of the antigen structure and the balance between humoral and cell-mediated immunity in rats. Clin Exp Immunol. 1977 May;28(2):280–288. [PMC free article] [PubMed] [Google Scholar]
- Drexhage H. A., van de Plassche E. M., Kokjé M., Leezenberg H. A. Abnormalities in cell-mediated immune functions to Haemophilus influenzae chronic purulent infections of the upper respiratory tract. Clin Immunol Immunopathol. 1983 Aug;28(2):218–228. doi: 10.1016/0090-1229(83)90156-3. [DOI] [PubMed] [Google Scholar]
- Fleer A., van der Hart M., Blok-Schut B. J., Schellekens P. T. Correlation of PPD and BCG-induced leukocyte migration inhibition, delayed cutaneous hypersensitivity, lymphocyte transformation in vitro and humoral antibodies to PPD in man. Eur J Immunol. 1976 Mar;6(3):163–167. doi: 10.1002/eji.1830060305. [DOI] [PubMed] [Google Scholar]
- GEORGE M., VAUGHAN J. H. In vitro cell migration as a model for delayed hypersensitivity. Proc Soc Exp Biol Med. 1962 Nov;111:514–521. doi: 10.3181/00379727-111-27841. [DOI] [PubMed] [Google Scholar]
- Graybill J. R., Alford R. H. Variability of sequential studies of lymphocyte blastogenesis in normal adults. Clin Exp Immunol. 1976 Jul;25(1):28–35. [PMC free article] [PubMed] [Google Scholar]
- Górski A. J. Superiority of corpuscular BCG to soluble PPD antigen in the leucocyte migration assay. Clin Exp Immunol. 1974 Sep;18(1):149–153. [PMC free article] [PubMed] [Google Scholar]
- Harrington J. T., Jr, Stastny P. Macrophage migration from an agarose droplet: development of a micromethod for assay of delayed hypersensitivity. J Immunol. 1973 Mar;110(3):752–759. [PubMed] [Google Scholar]
- Holt P. G., Fimmel P. J., Bartholomaeus W. N., Roberts L. M., Tandon M. K., Keast D. Dissociation of correlates of cellular immunity in man: functional heterogeneity within the antigen-reactive cell population? Int Arch Allergy Appl Immunol. 1976;51(5):560–573. doi: 10.1159/000231629. [DOI] [PubMed] [Google Scholar]
- Lewinski U. H., Mavlight G. M., Gutterman J. U., Hersh E. M. Interaction between repeated skin testing with recall antigens and temporal fluctuations of in vitro lymphocyte blastogenesis in cancer patients. Clin Immunol Immunopathol. 1977 Jan;7(1):77–84. doi: 10.1016/0090-1229(77)90032-0. [DOI] [PubMed] [Google Scholar]
- Nilsson B. S., Möller G. Reactivity of human lymphocytes to aggregated and nonaggregated PPD-tuberculin. Cell Immunol. 1972 Dec;5(4):555–560. doi: 10.1016/0008-8749(72)90105-0. [DOI] [PubMed] [Google Scholar]
- Roupe G., Strannegård O. The influence of antibody on the induction and elicitation of allergic contact dermatitis. Int Arch Allergy Appl Immunol. 1972;43(5):691–699. doi: 10.1159/000230885. [DOI] [PubMed] [Google Scholar]
- Räsänen L. Skin test reactivity and in vitro responses to microbes and microbial antigens. Clin Exp Immunol. 1980 Jun;40(3):566–572. [PMC free article] [PubMed] [Google Scholar]
- Senyk G., Hadley W. K. In vitro correlates of delayed hypersensitivity in man: ambiguity of polymorphonuclear neutrophils as indicator cells in leukocyte migration test. Infect Immun. 1973 Sep;8(3):370–380. doi: 10.1128/iai.8.3.370-380.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soborg M., Bendixen G. Human lymphocyte migration as a parameter of hypersensitivity. Acta Med Scand. 1967 Feb;181(2):247–256. doi: 10.1111/j.0954-6820.1967.tb07255.x. [DOI] [PubMed] [Google Scholar]
- Thestrup-Pedersen K. Temporary suppression of lymphocyte transformation after tuberculin skin testing. Immunology. 1974 Dec;27(6):965–971. [PMC free article] [PubMed] [Google Scholar]
- Thor D. E., Jureziz R. E., Veach S. R., Miller E., Dray S. Cell migration inhibition factor released by antigen from human peripheral lymphocytes. Nature. 1968 Aug 17;219(5155):755–757. doi: 10.1038/219755a0. [DOI] [PubMed] [Google Scholar]
- Tosca N., Parker D., Turk J. L. The effect of a delayed hypersensitivity skin reaction on in vitro parameters of cell-mediated immunity. Cell Immunol. 1981 Jul 15;62(1):28–37. doi: 10.1016/0008-8749(81)90296-3. [DOI] [PubMed] [Google Scholar]
- Valdimarsson H., Holt L., Riches H. R., Hobbs J. R. Lymphocyte abnormality in chronic mucocutaneous candidiasis. Lancet. 1970 Jun 13;1(7659):1259–1261. doi: 10.1016/s0140-6736(70)91742-3. [DOI] [PubMed] [Google Scholar]
- Van de Plassche-Boers E. M., Drexhage H. A., Kokjé-Kleingeld M. The use of somatic antigen of Haemophilus influenzae for the monitoring of T cell-mediated skin test reactivity in man. J Immunol Methods. 1985 Nov 7;83(2):353–361. doi: 10.1016/0022-1759(85)90257-1. [DOI] [PubMed] [Google Scholar]
- Virelizier J. L., Lenoir G., Griscelli C. Persistent Epstein-Barr virus infection in a child with hypergammaglobulinaemia and immunoblastic proliferation associated with a selective defect in immune interferon secretion. Lancet. 1978 Jul 29;2(8083):231–234. doi: 10.1016/s0140-6736(78)91744-0. [DOI] [PubMed] [Google Scholar]
- Weiser W. Y., Remold H. G., Block L. H., David J. R. Dissociation of human interferon-gamma-like activity from migration-inhibition factor. Cell Immunol. 1984 Oct 1;88(1):109–122. doi: 10.1016/0008-8749(84)90056-x. [DOI] [PubMed] [Google Scholar]
- Wysocki L. J., Sato V. L. "Panning" for lymphocytes: a method for cell selection. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2844–2848. doi: 10.1073/pnas.75.6.2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zabriskie J. B., Falk R. E. In vitro reactivity of lymphocytes to particulate and soluble antigens. Nature. 1970 Jun 6;226(5249):943–945. doi: 10.1038/226943a0. [DOI] [PubMed] [Google Scholar]


