Abstract
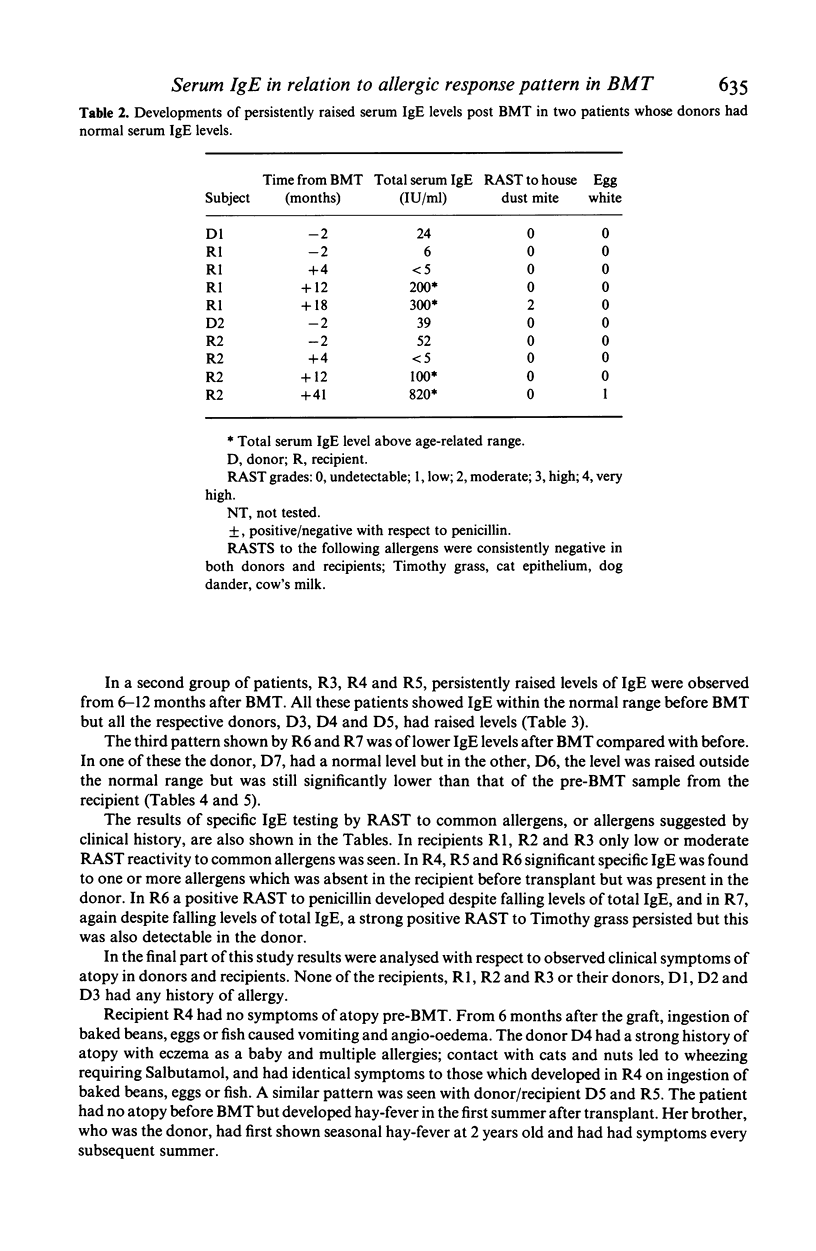
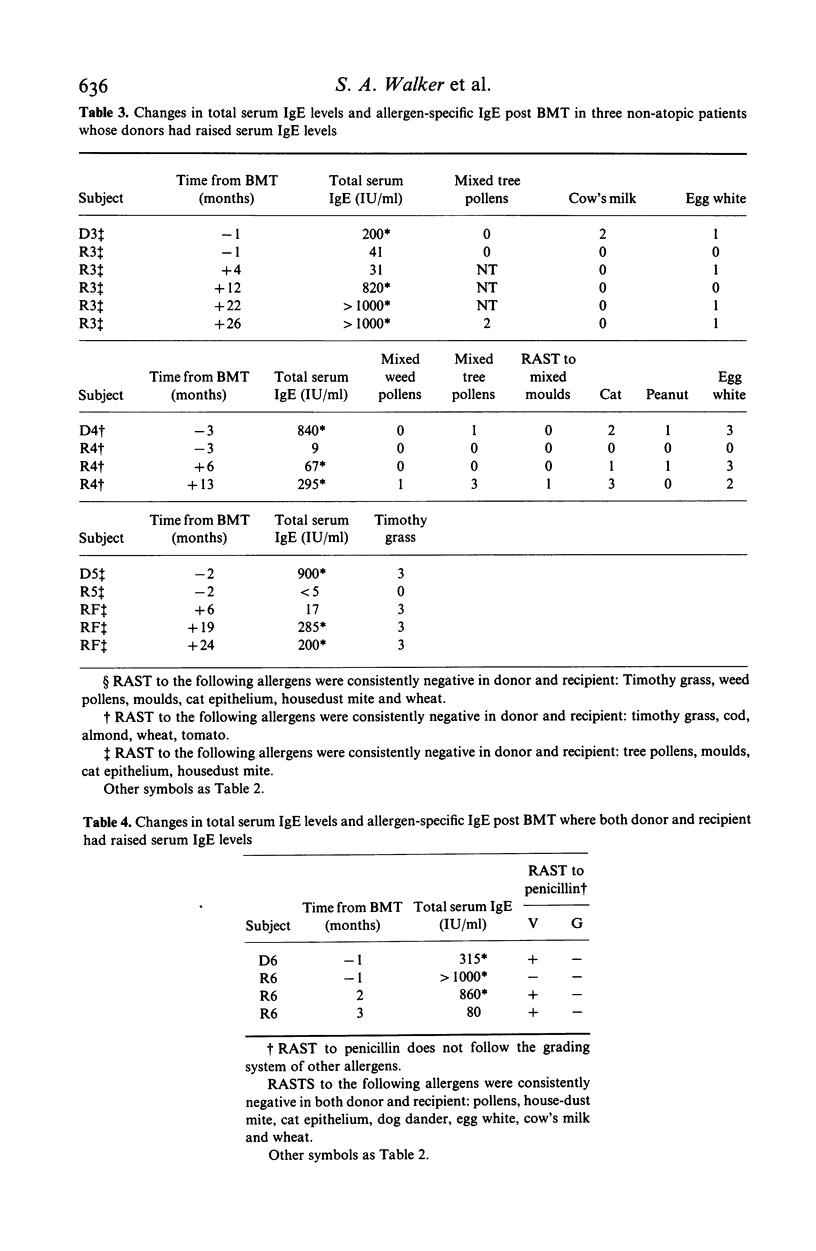
Total and allergen-specific serum IgE were measured in relation to allergic response pattern before and after bone marrow transplant (BMT) in seven sibling donor/recipient pairs. Two non-atopic recipients developed persistently raised total serum IgE levels but no apparent allergic response after BMT from non-atopic donors; three non-atopic recipients showed raised total serum IgE after BMT, with allergen-specific IgE to the same allergens as their respective atopic donors. A penicillin-tolerant recipient showed clinical sensitivity and specific IgE to penicillin after BMT from a penicillin-sensitive donor, but with this case both donor and recipient showed raised serum IgE levels. One atopic recipient showed decreased total IgE after BMT from a mildly atopic donor. These allergic response patterns could occur as a result of repopulation in the recipient with IgE-specific T lymphocytes having similar regulatory influences as in the donor. The pattern of acquired responses would also be consistent with reconstitution by primed B lymphocytes of donor origin.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Butler M., Atherton D., Levinsky R. J. Quantitative and functional deficit of suppressor T cells in children with atopic eczema. Clin Exp Immunol. 1982 Oct;50(1):92–98. [PMC free article] [PubMed] [Google Scholar]
- Byrom N. A., Caballero F., Campbell M. A., Chooi M., Lane A. M., Hugh-Jones K., Timlin D. M., Hobbs J. R. T-cell depletion and in vitro thymosin inducibility in asthmatic children. Clin Exp Immunol. 1978 Mar;31(3):490–498. [PMC free article] [PubMed] [Google Scholar]
- Carney W. P., Iacoviello V., Hirsch M. S. Functional properties of T lymphocytes and their subsets in cytomegalovirus mononucleosis. J Immunol. 1983 Jan;130(1):390–393. [PubMed] [Google Scholar]
- Di Padova F., Gratwohl A., Corneo M., Speck B. Functional and morphological suppressor T cell deficit in acute GVHD. Clin Exp Immunol. 1984 Dec;58(3):611–618. [PMC free article] [PubMed] [Google Scholar]
- Hamaoka T., Katz D. H., Benacerraf B. Hapten-specific IgE antibody responses in mice. II. Cooperative interactions between adoptively transferred T and B lymphocytes in the development of IgE response. J Exp Med. 1973 Sep 1;138(3):538–556. doi: 10.1084/jem.138.3.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamaoka T., Katz D. H., Bloch K. J., Benacerraf B. Hapten-specific IgE antibody responses in mice. I. Secondary IgE responses in irradiated recipients of syngeneic primed spleen cells. J Exp Med. 1973 Jul 1;138(1):306–311. doi: 10.1084/jem.138.1.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz D. H. Regulation of the IgE system: experimental and clinical aspects. Allergy. 1984 Feb;39(2):81–106. doi: 10.1111/j.1398-9995.1984.tb01940.x. [DOI] [PubMed] [Google Scholar]
- Leung D. Y., Geha R. S. Regulation of IGE synthesis in man. Clin Immunol Rev. 1984;3(1):1–24. [PubMed] [Google Scholar]
- Lum L. G., Seigneuret M. C., Storb R. F., Witherspoon R. P., Thomas E. D. In vitro regulation of immunoglobulin synthesis after marrow transplantation. I. T-cell and B-cell deficiencies in patients with and without chronic graft-versus-host disease. Blood. 1981 Sep;58(3):431–439. [PubMed] [Google Scholar]
- Marsh D. G., Bias W. B., Ishizaka K. Genetic control of basal serum immunoglobulin E level and its effect on specific reaginic sensitivity. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3588–3592. doi: 10.1073/pnas.71.9.3588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrett T. G., Merrett J. The RAST principle and the use of mixed-allergen RAST as a screening test for IgE-mediated allergies. Methods Enzymol. 1980;70(A):376–387. doi: 10.1016/s0076-6879(80)70065-4. [DOI] [PubMed] [Google Scholar]
- Mitsuya H., Sato M., Hirano T., Fujimoto K., Kawano F., Kishimoto S. Evidence for a malignant proliferation of IgE-class specific helper T cells in a patient with Sézary syndrome exhibiting massive hyperimmunoglobulinemia E. Clin Immunol Immunopathol. 1983 Feb;26(2):171–183. doi: 10.1016/0090-1229(83)90135-6. [DOI] [PubMed] [Google Scholar]
- Reinherz E. L., Parkman R., Rappeport J., Rosen F. S., Schlossman S. F. Aberrations of suppressor T cells in human graft-versus-host disease. N Engl J Med. 1979 May 10;300(19):1061–1068. doi: 10.1056/NEJM197905103001901. [DOI] [PubMed] [Google Scholar]
- Saarinen U. M. Transfer of latent atopy by bone marrow transplantation? A case report. J Allergy Clin Immunol. 1984 Aug;74(2):196–200. doi: 10.1016/0091-6749(84)90286-0. [DOI] [PubMed] [Google Scholar]
- Vaz N. M., Levine B. B. Immune responses of inbred mice to repeated low doses of antigen: relationship to histocompatibility (H-2) type. Science. 1970 May 15;168(3933):852–854. doi: 10.1126/science.168.3933.852. [DOI] [PubMed] [Google Scholar]
- Walker S. A., Rogers T. R., Perry D., Hobbs J. R., Riches P. G. Increased serum IgE concentrations during infection and graft versus host disease after bone marrow transplantation. J Clin Pathol. 1984 Apr;37(4):460–462. doi: 10.1136/jcp.37.4.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witherspoon R. P., Deeg H. J., Lum L. G., Ochs H. D., Hansen J. A., Thomas E. D., Storb R. Immunologic recovery in human marrow graft recipients given cyclosporine or methotrexate for the prevention of graft-versus-host disease. Transplantation. 1984 May;37(5):456–461. doi: 10.1097/00007890-198405000-00007. [DOI] [PubMed] [Google Scholar]
- Witherspoon R. P., Lum L. G., Storb R., Thomas E. D. In vitro regulation of immunoglobulin synthesis after human marrow transplantation. II. Deficient T and non-T lymphocyte function within 3-4 months of allogeneic, syngeneic, or autologous marrow grafting for hematologic malignancy. Blood. 1982 Apr;59(4):844–850. [PubMed] [Google Scholar]
- Wybran J., Fudenberg H. H. Thymus-derived rosette-forming cells in various human disease states: cancer, lymphoma, bacterial and viral infections, and other diseases. J Clin Invest. 1973 May;52(5):1026–1032. doi: 10.1172/JCI107267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamura M., Newton R. C., James D. C., Humble J. G., Butler L. J., Hobbs J. R. Uncomplicated HL-A matched sibling bone marrow graft for combined immune deficiency. Br Med J. 1972 Apr 29;2(5808):265–269. doi: 10.1136/bmj.2.5808.265. [DOI] [PMC free article] [PubMed] [Google Scholar]


