Figure 4.
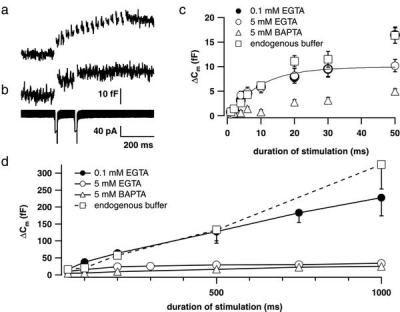
Kinetics of exocytosis in IHCs. (a) Recordings (n = 10) of ΔCm responses to trains of 15 depolarizations to −15 mV for 10 ms (20-ms interval) were averaged (scaling as in b). The first depolarization caused the largest response. (b) A paired-pulse paradigm (two 15-ms depolarizations to −15 mV; 100-ms interpulse interval) resulted in a larger first than second ΔCm (Upper). Secretory depression occurred, although Ca2+ currents were nearly identical (Lower). Data represent an average of four responses. (a and b) Experiments were performed in the perforated patch-configuration. (c) ΔCm triggered by depolarizations of different durations to −15 mV plotted against pulse duration up to 50 ms to emphasize the fast secretory component. Experiments were performed in perforated-patch (squares; n = 25) or whole-cell configuration, and then Ca2+ chelators were introduced to the IHC's cytosol as specified. Mixtures of equal amounts of Ca2+-loaded and Ca2+-free chelators were used to avoid possible depriming effects of very low [Ca2+]i levels. Stimulation was started 60 s after establishing the whole-cell configuration. Filled circles, low buffering capacity (0.1 mM Ca2+-free EGTA/0.1 Ca2+-EGTA; n = 33 cells); open circles, high buffering capacity, slower Ca2+-binding (5 mM Ca2+-free EGTA/5 mM Ca2+-EGTA; n = 26 cells); triangles, high buffering capacity, faster Ca2+-binding (5 mM Ca2+-free BAPTA/5 mM Ca2+-BAPTA; n = 16 cells); solid line, exponential fit to the first 50 ms of the “high EGTA” data. (d) Same data set as in c. Slow Cm rise during continued depolarization.
