Abstract
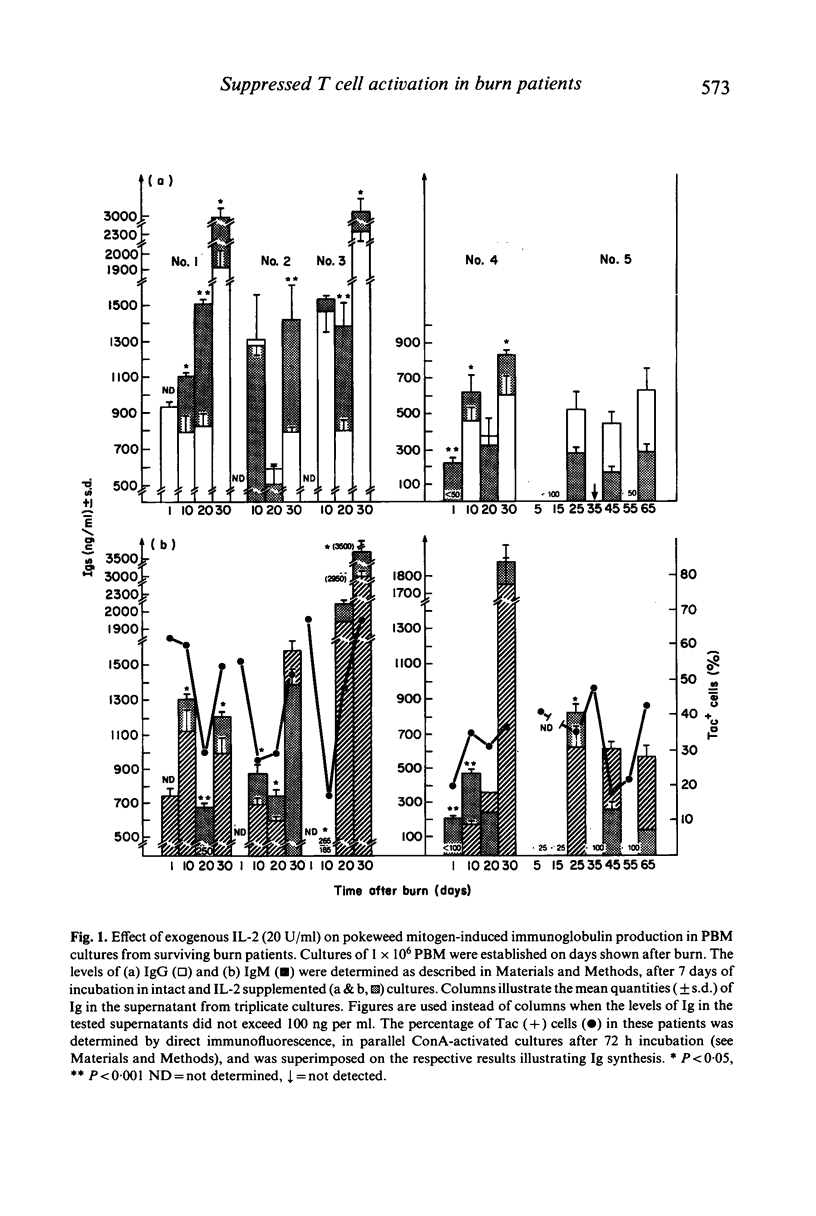
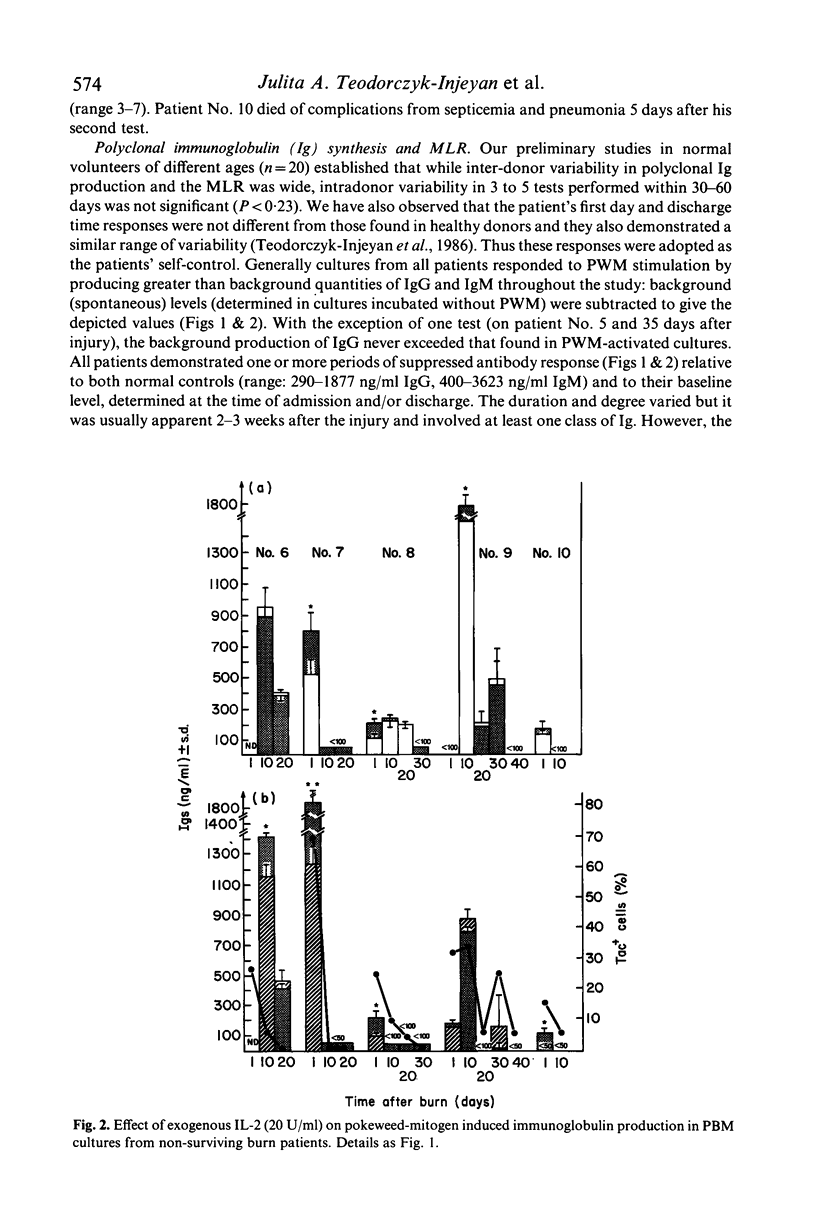
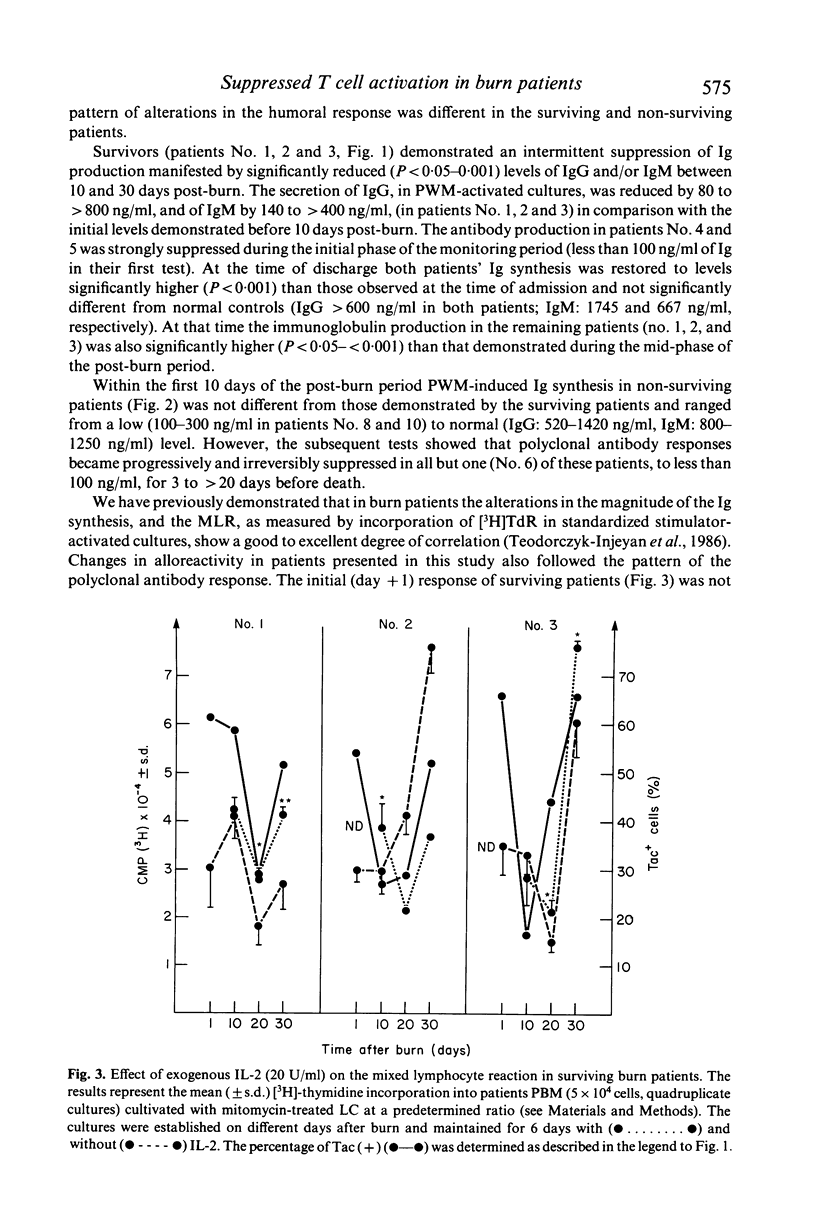
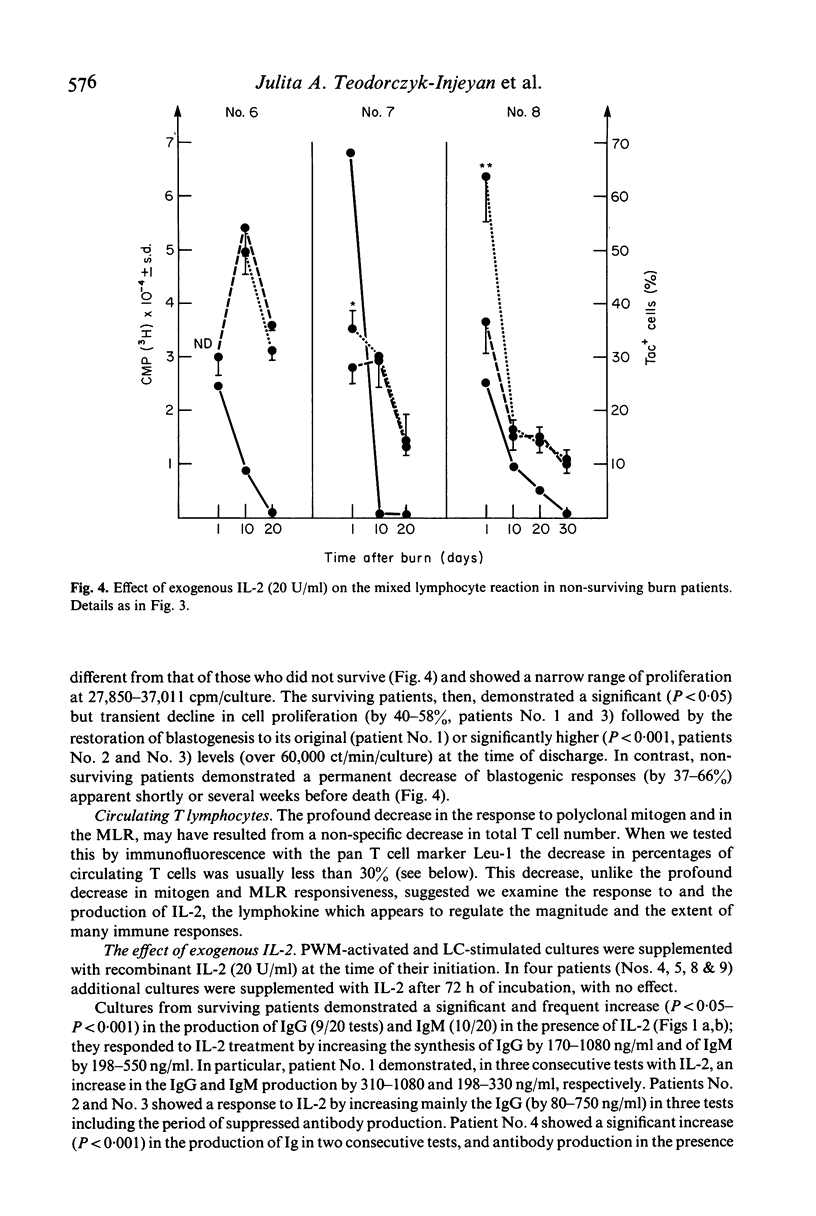
In the burn patient, the mechanisms leading to impaired T lymphocyte activity are unclear. The capacity for T cell proliferation and the expression of Tac antigen (IL-2 receptor) was assessed during the post-burn period in patients with injuries ranging from 5-68% total body surface area. T cell-dependent (polyclonal) immunoglobulin synthesis, mixed lymphocyte reaction and Interleukin-2 production were also determined in these patients and correlated with survival. Surviving patients demonstrated a transient reduction while terminal patients exhibited a permanent reduction in the number of Tac (+) lymphocytes, unrelated to the absolute number of T cells, during the post-burn period. The reduced percentage of IL-2 receptor-expressing T cells coincided with the suppressed antibody response and reduced alloreactivity. Although the concentration of IL-2 was decreased in all patients throughout the hospitalization period, surviving patients showed a gradual increase in its production while terminal patients gradually decreased to undetectable levels. Exogenous recombinant IL-2 induced a significant enhancement of in-vitro polyclonal immunoglobulin production and blastogenesis in the mixed lymphocyte reaction in immunosuppressed patients who demonstrated up to 50% reduction in the percentage of IL-2 receptor positive cells. Thus, the reduced capacity for production of and response to IL-2 after thermal injury may lead to the immunosuppression due to a lack of T lymphocyte clonal expansion. The permanent nature of this defect in patients who died from fatal sepsis may suggest a causative relationship.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acheson E. D., Winter P. D., Hadfield E., Macbeth R. G. Is nasal adenocarcinoma in the Buckinghamshire furniture industry declining? Nature. 1982 Sep 16;299(5880):263–265. doi: 10.1038/299263a0. [DOI] [PubMed] [Google Scholar]
- Antonacci A. C., Calvano S. E., Reaves L. E., Prajapati A., Bockman R., Welte K., Mertelsmann R., Gupta S., Good R. A., Shires G. T. Autologous and allogeneic mixed-lymphocyte responses following thermal injury in man: the immunomodulatory effects of interleukin 1, interleukin 2, and a prostaglandin inhibitor, WY-18251. Clin Immunol Immunopathol. 1984 Feb;30(2):304–320. doi: 10.1016/0090-1229(84)90064-3. [DOI] [PubMed] [Google Scholar]
- Antonacci A. C., Good R. A., Gupta S. T-cell subpopulations following thermal injury. Surg Gynecol Obstet. 1982 Jul;155(1):1–8. [PubMed] [Google Scholar]
- Bleackley R. C., Havele C., Paetkau V. Cellular and molecular properties of an antigen-specific cytotoxic T lymphocyte line. J Immunol. 1982 Feb;128(2):758–767. [PubMed] [Google Scholar]
- Cantrell D. A., Smith K. A. The interleukin-2 T-cell system: a new cell growth model. Science. 1984 Jun 22;224(4655):1312–1316. doi: 10.1126/science.6427923. [DOI] [PubMed] [Google Scholar]
- Cantrell D. A., Smith K. A. Transient expression of interleukin 2 receptors. Consequences for T cell growth. J Exp Med. 1983 Dec 1;158(6):1895–1911. doi: 10.1084/jem.158.6.1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chouaib S., Chatenoud L., Klatzmann D., Fradelizi D. The mechanisms of inhibition of human IL 2 production. II. PGE2 induction of suppressor T lymphocytes. J Immunol. 1984 Apr;132(4):1851–1857. [PubMed] [Google Scholar]
- Constantian M. B. Association of sepsis with an immunosuppressive polypeptide in the serum of burn patients. Ann Surg. 1978 Aug;188(2):209–215. doi: 10.1097/00000658-197808000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curreri P. W., Richmond D., Marvin J., Baxter C. R. Dietary requirements of patients with major burns. J Am Diet Assoc. 1974 Oct;65(4):415–417. [PubMed] [Google Scholar]
- Devos R., Plaetinck G., Cheroutre H., Simons G., Degrave W., Tavernier J., Remaut E., Fiers W. Molecular cloning of human interleukin 2 cDNA and its expression in E. coli. Nucleic Acids Res. 1983 Jul 11;11(13):4307–4323. doi: 10.1093/nar/11.13.4307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillis S. Interleukin 2: biology and biochemistry. J Clin Immunol. 1983 Jan;3(1):1–13. doi: 10.1007/BF00919133. [DOI] [PubMed] [Google Scholar]
- Günther J., Haas W., Von Boehmer H. Suppression of T cell responses through competition for T cell growth factor (interleukin 2). Eur J Immunol. 1982 Mar;12(3):247–249. doi: 10.1002/eji.1830120315. [DOI] [PubMed] [Google Scholar]
- Hirano T., Kuritani T., Kishimoto T., Yamamura Y. In vitro immune response of human peripheral lymphocytes. I. The mechanism(s) involved in T cell helper functions in the pokeweed mitogen-induced differentiation and proliferation of B cells. J Immunol. 1977 Oct;119(4):1235–1241. [PubMed] [Google Scholar]
- Keightley R. G., Cooper M. D., Lawton A. R. The T cell dependence of B cell differentiation induced by pokeweed mitogen. J Immunol. 1976 Nov;117(5 Pt 1):1538–1544. [PubMed] [Google Scholar]
- Kondo N., Orii T., Uetake H. Competence of B cells for T-cell help in pokeweed mitogen-induced immunoglobulin production. Clin Immunol Immunopathol. 1983 Feb;26(2):192–200. doi: 10.1016/0090-1229(83)90137-x. [DOI] [PubMed] [Google Scholar]
- Larsson E. L. Mechanism of T cell activation. II. Antigen- and lectin-dependent acquisition of responsiveness to TCGF is a nonmitogenic, active response of resting T cells. J Immunol. 1981 Apr;126(4):1323–1326. [PubMed] [Google Scholar]
- Miller C. L., Baker C. C. Changes in lymphocyte activity after thermal injury. The role of suppressor cells. J Clin Invest. 1979 Feb;63(2):202–210. doi: 10.1172/JCI109290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyawaki T., Yachie A., Uwadana N., Ohzeki S., Nagaoki T., Taniguchi N. Functional significance of Tac antigen expressed on activated human T lymphocytes: Tac antigen interacts with T cell growth factor in cellular proliferation. J Immunol. 1982 Dec;129(6):2474–2478. [PubMed] [Google Scholar]
- Moretta A. Frequency and surface phenotype of human T lymphocytes producing interleukin 2. Analysis by limiting dilution and cell cloning. Eur J Immunol. 1985 Feb;15(2):148–155. doi: 10.1002/eji.1830150208. [DOI] [PubMed] [Google Scholar]
- Munster A. M. Post-traumatic immunosuppression is due to activation of suppressor T cells. Lancet. 1976 Jun 19;1(7973):1329–1330. doi: 10.1016/s0140-6736(76)92658-1. [DOI] [PubMed] [Google Scholar]
- Munster A. M., Winchurch R. A., Birmingham W. J., Keeling P. Longitudinal assay of lymphocyte responsiveness in patients with major burns. Ann Surg. 1980 Dec;192(6):772–775. doi: 10.1097/00000658-198012000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neilan B. A., Taddeini L., Strate R. G. T lymphocyte rosette formation after major burns. JAMA. 1977 Aug 8;238(6):493–496. [PubMed] [Google Scholar]
- Ninnemann J. L., Stein M. D. Bacterial endotoxin and the generation of suppressor T cells following thermal injury. J Trauma. 1980 Nov;20(11):959–966. doi: 10.1097/00005373-198011000-00010. [DOI] [PubMed] [Google Scholar]
- Ninnemann J. L., Stockland A. E., Condie J. T. Induction of prostaglandin synthesis-dependent suppressor cells with endotoxin: occurrence in patients with thermal injuries. J Clin Immunol. 1983 Apr;3(2):142–150. doi: 10.1007/BF00915485. [DOI] [PubMed] [Google Scholar]
- O'Mahony J. B., Palder S. B., Wood J. J., McIrvine A., Rodrick M. L., Demling R. H., Mannick J. A. Depression of cellular immunity after multiple trauma in the absence of sepsis. J Trauma. 1984 Oct;24(10):869–875. doi: 10.1097/00005373-198410000-00001. [DOI] [PubMed] [Google Scholar]
- Ozkan A. N., Ninnemann J. L. Circulating mediators in thermal injuries: isolation and characterization of a burn injury-induced immunosuppressive serum component. J Burn Care Rehabil. 1985 Mar-Apr;6(2):147–151. [PubMed] [Google Scholar]
- Palacios R., Möller G. T cell growth factor abrogates concanavalin A-induced suppressor cell function. J Exp Med. 1981 May 1;153(5):1360–1365. doi: 10.1084/jem.153.5.1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reem G. H., Yeh N. H. Interleukin 2 regulates expression of its receptor and synthesis of gamma interferon by human T lymphocytes. Science. 1984 Jul 27;225(4660):429–430. doi: 10.1126/science.6429853. [DOI] [PubMed] [Google Scholar]
- Rijkers G. T., Mosier D. E. Pneumococcal polysaccharides induce antibody formation by human B lymphocytes in vitro. J Immunol. 1985 Jul;135(1):1–4. [PubMed] [Google Scholar]
- Robb R. J., Greene W. C., Rusk C. M. Low and high affinity cellular receptors for interleukin 2. Implications for the level of Tac antigen. J Exp Med. 1984 Oct 1;160(4):1126–1146. doi: 10.1084/jem.160.4.1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robb R. J., Munck A., Smith K. A. T cell growth factor receptors. Quantitation, specificity, and biological relevance. J Exp Med. 1981 Nov 1;154(5):1455–1474. doi: 10.1084/jem.154.5.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roifman C. M., Mills G. B., Chu M., Gelfand E. W. Functional comparison of recombinant interleukin 2 (IL-2) with IL-2-containing preparations derived from cultured cells. Cell Immunol. 1985 Oct 1;95(1):146–156. doi: 10.1016/0008-8749(85)90303-x. [DOI] [PubMed] [Google Scholar]
- Schreier M. H., Iscove N. N., Tees R., Aarden L., von Boehmer H. Clones of killer and helper T cells: growth requirements, specificity and retention of function in long-term culture. Immunol Rev. 1980;51:315–336. doi: 10.1111/j.1600-065x.1980.tb00326.x. [DOI] [PubMed] [Google Scholar]
- Smith K. A., Cantrell D. A. Interleukin 2 regulates its own receptors. Proc Natl Acad Sci U S A. 1985 Feb;82(3):864–868. doi: 10.1073/pnas.82.3.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood G. W., Volenec F. J., Mani M. M., Humphrey L. J. Dynamics of T-lymphocyte subpopulations and T-lymphocyte function following thermal injury. Clin Exp Immunol. 1978 Feb;31(2):291–297. [PMC free article] [PubMed] [Google Scholar]
- Wood J. J., Rodrick M. L., O'Mahony J. B., Palder S. B., Saporoschetz I., D'Eon P., Mannick J. A. Inadequate interleukin 2 production. A fundamental immunological deficiency in patients with major burns. Ann Surg. 1984 Sep;200(3):311–320. doi: 10.1097/00000658-198409000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]



