Abstract
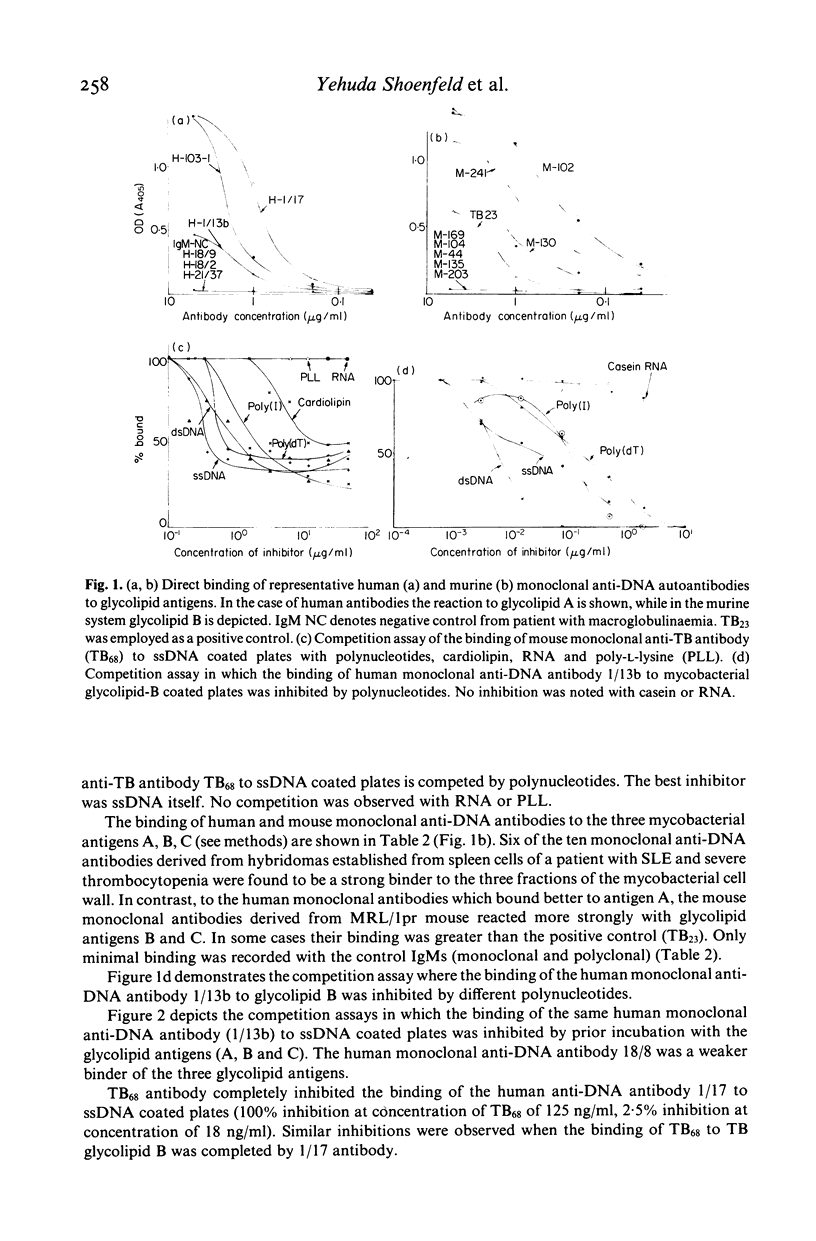
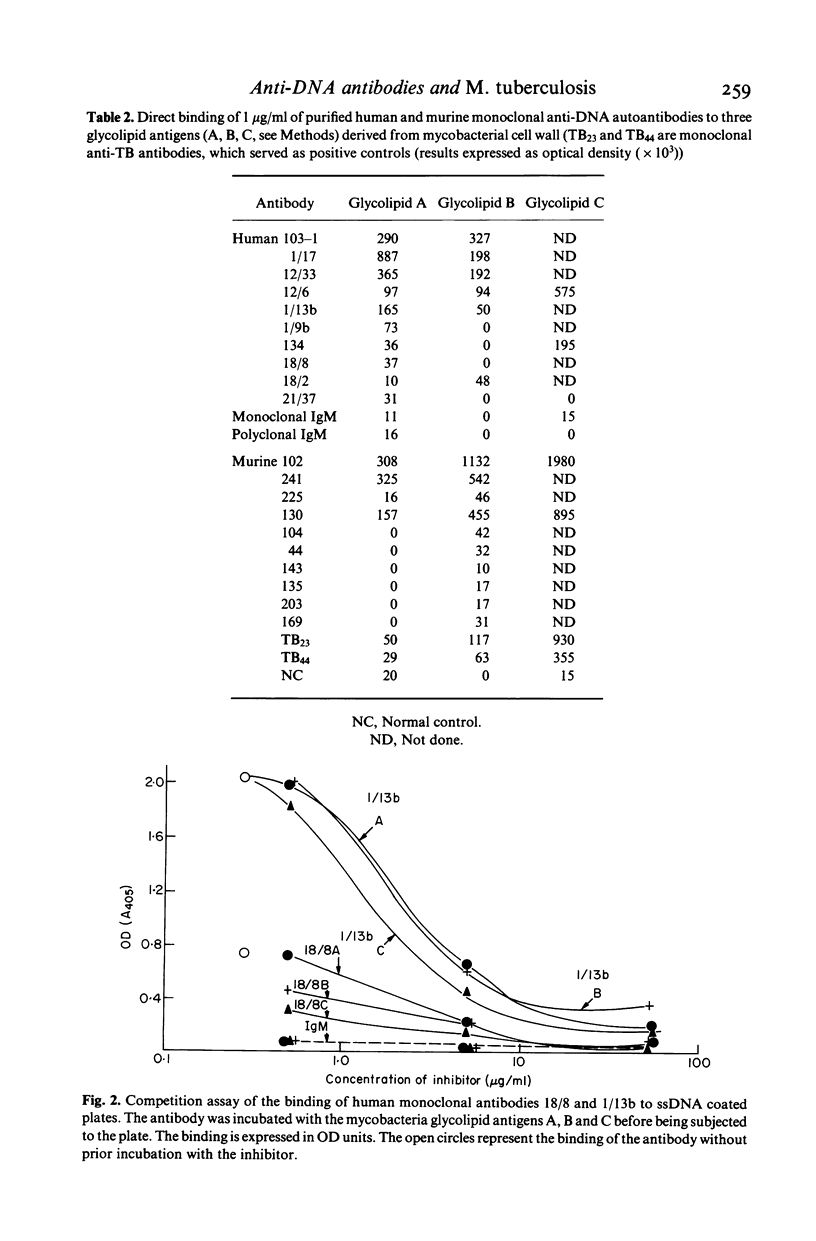
Classical models of experimental autoimmune diseases, such as adjuvant arthritis entail the use of mycobacteria. Furthermore, BCG immunotherapy may be followed by arthritic symptoms. To test the infection-autoimmunity relationship of mycobacteria, we used monoclonal antibodies raised against M. tuberculosis and against DNA. Murine monoclonal anti-TB antibodies were found to react with ssDNA, dsDNA and other polynucleotides. Monoclonal anti-DNA autoantibodies derived from patients and mice with SLE bound to three glycolipids shared among all mycobacteria and derived from mycobacterial cell wall. Prior incubation of the antibodies with ssDNA and other polynucleotides or with glycolipid antigens inhibited binding. These results indicate that infecting mycobacteria share antigens with human tissue, thus accounting in part for the production of autoantibodies in mycobacterial infections.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrzejewski C., Jr, Stollar B. D., Lalor T. M., Schwartz R. S. Hybridoma autoantibodies to DNA. J Immunol. 1980 Mar;124(3):1499–1502. [PubMed] [Google Scholar]
- Bodansky H. J., Littlewood J. M., Bottazzo G. F., Dean B. M., Hambling M. H. Which virus causes the initial islet lesion in type 1 diabetes? Lancet. 1984 Feb 18;1(8373):401–402. doi: 10.1016/s0140-6736(84)90459-8. [DOI] [PubMed] [Google Scholar]
- Coates A. R., Hewitt J., Allen B. W., Ivanyi J., Mitchison D. A. Antigenic diversity of Mycobacterium tuberculosis and Mycobacterium bovis detected by means of monoclonal antibodies. Lancet. 1981 Jul 25;2(8239):167–169. doi: 10.1016/s0140-6736(81)90355-x. [DOI] [PubMed] [Google Scholar]
- Galvão-Castro B., Sá Ferreira J. A., Marzochi K. F., Marzochi M. C., Coutinho S. G., Lambert P. H. Polyclonal B cell activation, circulating immune complexes and autoimmunity in human american visceral leishmaniasis. Clin Exp Immunol. 1984 Apr;56(1):58–66. [PMC free article] [PubMed] [Google Scholar]
- Haspel M. V., Onodera T., Prabhakar B. S., Horita M., Suzuki H., Notkins A. L. Virus-induced autoimmunity: monoclonal antibodies that react with endocrine tissues. Science. 1983 Apr 15;220(4594):304–306. doi: 10.1126/science.6301002. [DOI] [PubMed] [Google Scholar]
- Isenberg D. A., Shoenfeld Y., Madaio M. P., Rauch J., Reichlin M., Stollar B. D., Schwartz R. S. Anti-DNA antibody idiotypes in systemic lupus erythematosus. Lancet. 1984 Aug 25;2(8400):417–422. doi: 10.1016/s0140-6736(84)92904-0. [DOI] [PubMed] [Google Scholar]
- Jacob L., Tron F., Bach J. F., Louvard D. A monoclonal anti-DNA antibody also binds to cell-surface protein(s). Proc Natl Acad Sci U S A. 1984 Jun;81(12):3843–3845. doi: 10.1073/pnas.81.12.3843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane D., Koprowski H. Molecular recognition and the future of monoclonal antibodies. Nature. 1982 Mar 18;296(5854):200–202. doi: 10.1038/296200a0. [DOI] [PubMed] [Google Scholar]
- Lindqvist K. J., Coleman R. E., Osterland C. K. Autoantibodies in chronic pulmonary tuberculosis. J Chronic Dis. 1970 Apr;22(11):717–725. doi: 10.1016/0021-9681(70)90047-0. [DOI] [PubMed] [Google Scholar]
- Murray H. W. Transient autoimmune hemolytic anemia and pulmonary tuberculosis. N Engl J Med. 1978 Aug 31;299(9):488–488. doi: 10.1056/nejm197808312990920. [DOI] [PubMed] [Google Scholar]
- Naparstek Y., Duggan D., Schattner A., Madaio M. P., Goni F., Frangione B., Stollar B. D., Kabat E. A., Schwartz R. S. Immunochemical similarities between monoclonal antibacterial Waldenstrom's macroglobulins and monoclonal anti-DNA lupus autoantibodies. J Exp Med. 1985 Jun 1;161(6):1525–1538. doi: 10.1084/jem.161.6.1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pangborn M. C., McKinney J. A. Purification of serologically active phosphoinositides of Mycobacterium tuberculosis. J Lipid Res. 1966 Sep;7(5):627–633. [PubMed] [Google Scholar]
- Paterson P. Y. Microbial parasitism cross-reactive with host antigen: implications concerning loss of "self" tolerance and development of autoimmune disease. Recomb DNA Tech Bull. 1981 Sep;4(3):98–107. [PubMed] [Google Scholar]
- Pettersson E., Törnroth T., Miettinen A. Simultaneous anti-glomerular basement membrane and membranous glomerulonephritis: case report and literature review. Clin Immunol Immunopathol. 1984 May;31(2):171–180. doi: 10.1016/0090-1229(84)90237-x. [DOI] [PubMed] [Google Scholar]
- Schwartz R. S., Stollar B. D. Origins of anti-DNA autoantibodies. J Clin Invest. 1985 Feb;75(2):321–327. doi: 10.1172/JCI111704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoenfeld Y., Hsu-Lin S. C., Gabriels J. E., Silberstein L. E., Furie B. C., Furie B., Stollar B. D., Schwartz R. S. Production of autoantibodies by human-human hybridomas. J Clin Invest. 1982 Jul;70(1):205–208. doi: 10.1172/JCI110595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoenfeld Y., Isenberg D. A., Rauch J., Madaio M. P., Stollar B. D., Schwartz R. S. Idiotypic cross-reactions of monoclonal human lupus autoantibodies. J Exp Med. 1983 Sep 1;158(3):718–730. doi: 10.1084/jem.158.3.718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoenfeld Y., Schwartz R. S. Immunologic and genetic factors in autoimmune diseases. N Engl J Med. 1984 Oct 18;311(16):1019–1029. doi: 10.1056/NEJM198410183111605. [DOI] [PubMed] [Google Scholar]
- Simpson R. W., McGinty L., Simon L., Smith C. A., Godzeski C. W., Boyd R. J. Association of parvoviruses with rheumatoid arthritis of humans. Science. 1984 Mar 30;223(4643):1425–1428. doi: 10.1126/science.6701529. [DOI] [PubMed] [Google Scholar]
- Weiss M., Ingbar S. H., Winblad S., Kasper D. L. Demonstration of a saturable binding site for thyrotropin in Yersinia enterocolitica. Science. 1983 Mar 18;219(4590):1331–1333. doi: 10.1126/science.6298936. [DOI] [PubMed] [Google Scholar]
- van Eden W., Holoshitz J., Nevo Z., Frenkel A., Klajman A., Cohen I. R. Arthritis induced by a T-lymphocyte clone that responds to Mycobacterium tuberculosis and to cartilage proteoglycans. Proc Natl Acad Sci U S A. 1985 Aug;82(15):5117–5120. doi: 10.1073/pnas.82.15.5117. [DOI] [PMC free article] [PubMed] [Google Scholar]


