Abstract
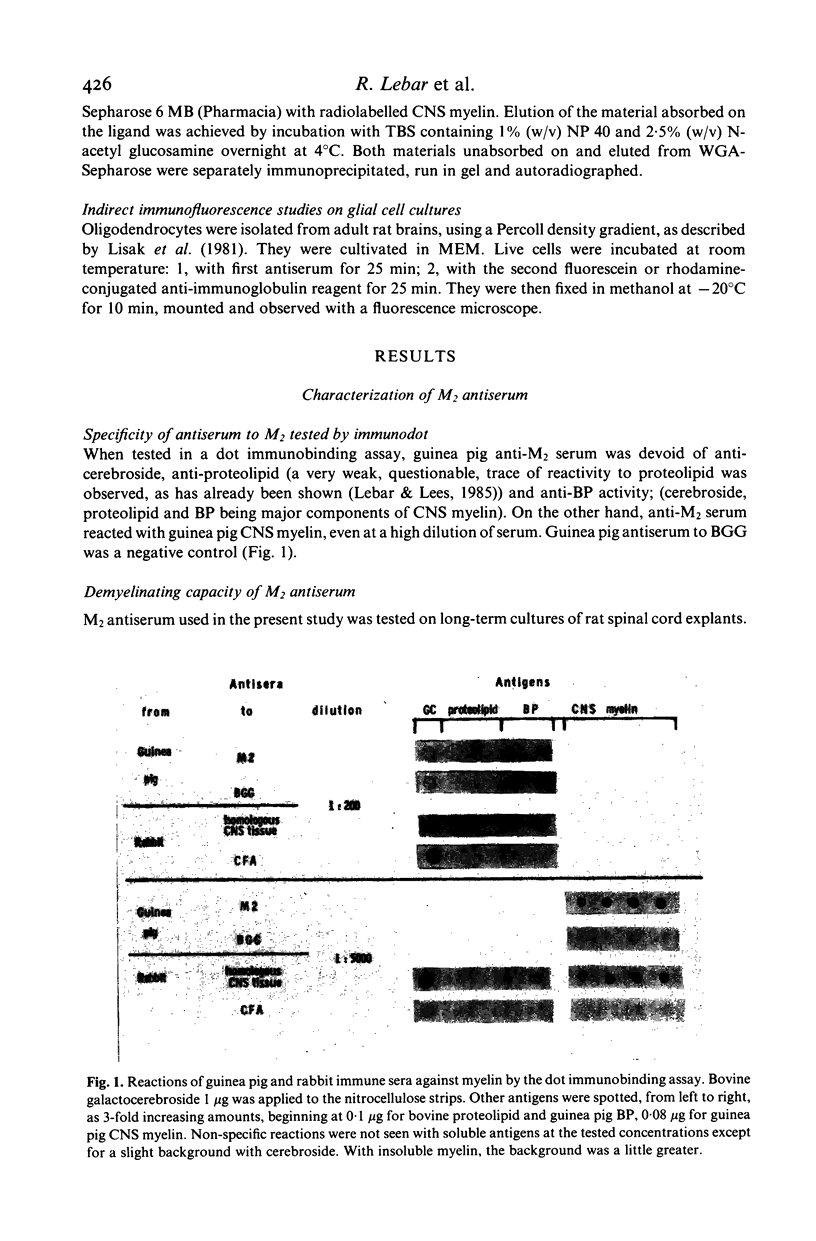
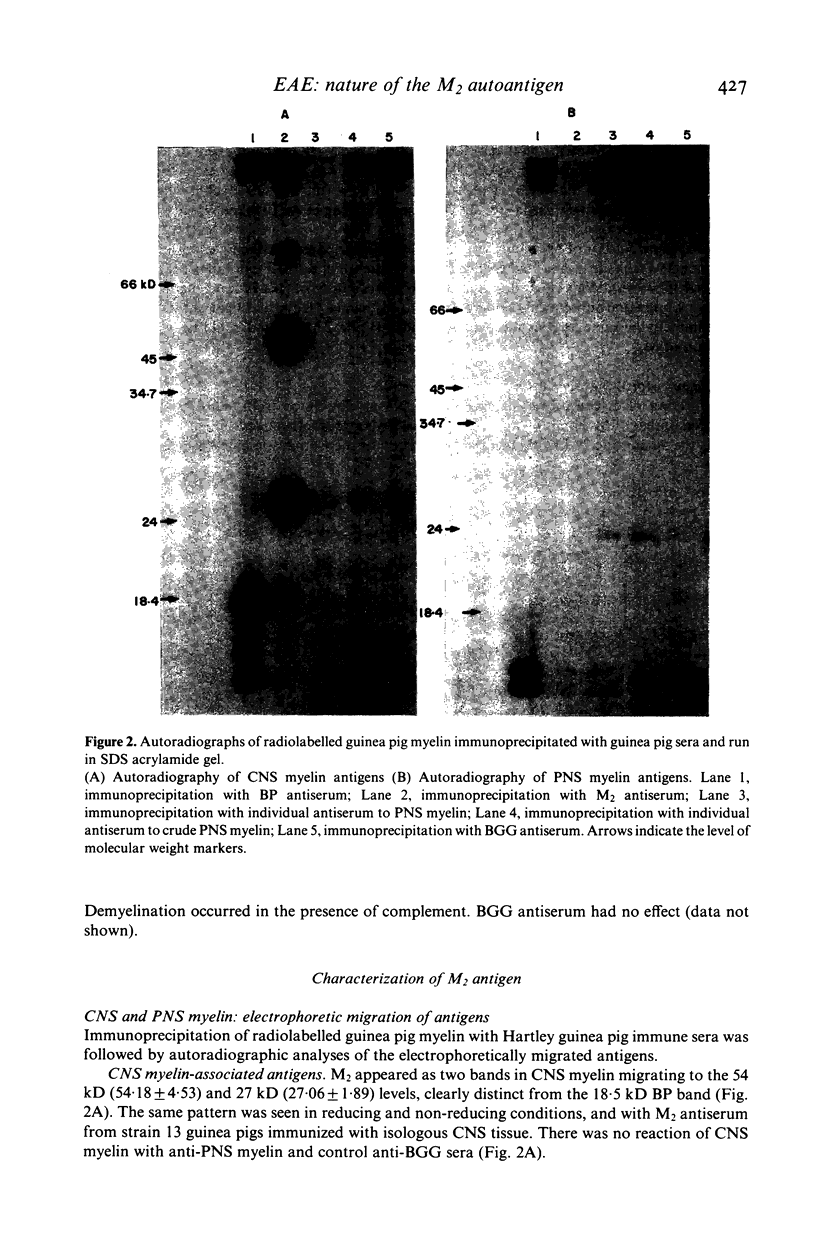
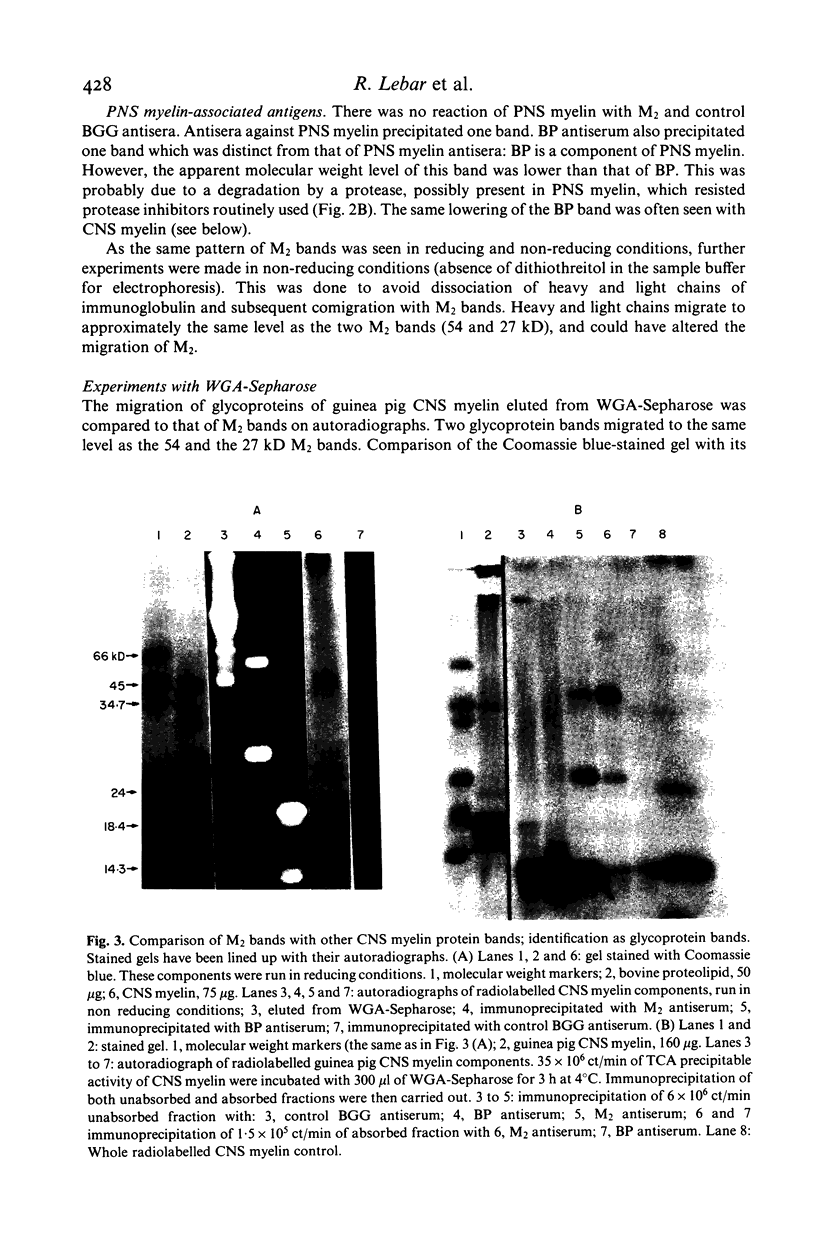
Autoantibodies with in-vitro demyelinating capacity induced in Hartley and strain 13 guinea pigs with homologous central nervous system (CNS) tissue were used to characterize the target autoantigen M2. Using the Dot Immunobinding technique, M2 was found to be a component of CNS myelin different from basic protein (BP) and from cerebroside. The expression of M2 on oligodendrocytes, cells known to produce CNS myelin, also confirmed that M2 was a component of CNS myelin. Furthermore, the autoradiography of immunoprecipitates formed with radiolabelled guinea pig myelin and analysed in sodium dodecyl sulphate gels showed that M2 was specific to CNS myelin and absent in peripheral nervous system (PNS) myelin. On electrophoresis M2 appeared as two CNS myelin protein bands at the 27 and 54 KD molecular weight levels, distinct from the major protein bands of proteolipid and BP. M2 bands were of glycoprotein nature, as was demonstrated by affinity chromatography of CNS myelin on wheat germ agglutinin (WGA)-Sepharose. A monoclonal antibody induced by BP-free CNS glycoproteins recognized the same bands as anti-M2 serum in guinea pig CNS myelin. This would imply that both M2 bands share common determinants. M2 bands similar to the above in guinea pig were also shown in rat, rabbit and bovine CNS myelin with guinea pig antibodies. The same type of anti-M2 antibodies were induced in rabbit immunized with homologous CNS tissue. Although only a minor component of myelin, M2 is strongly immunogenic compared to BP. M2 antigen could thus be the target of chronic demyelinating processes such as experimental allergic encephalomyelitis.
Full text
PDF


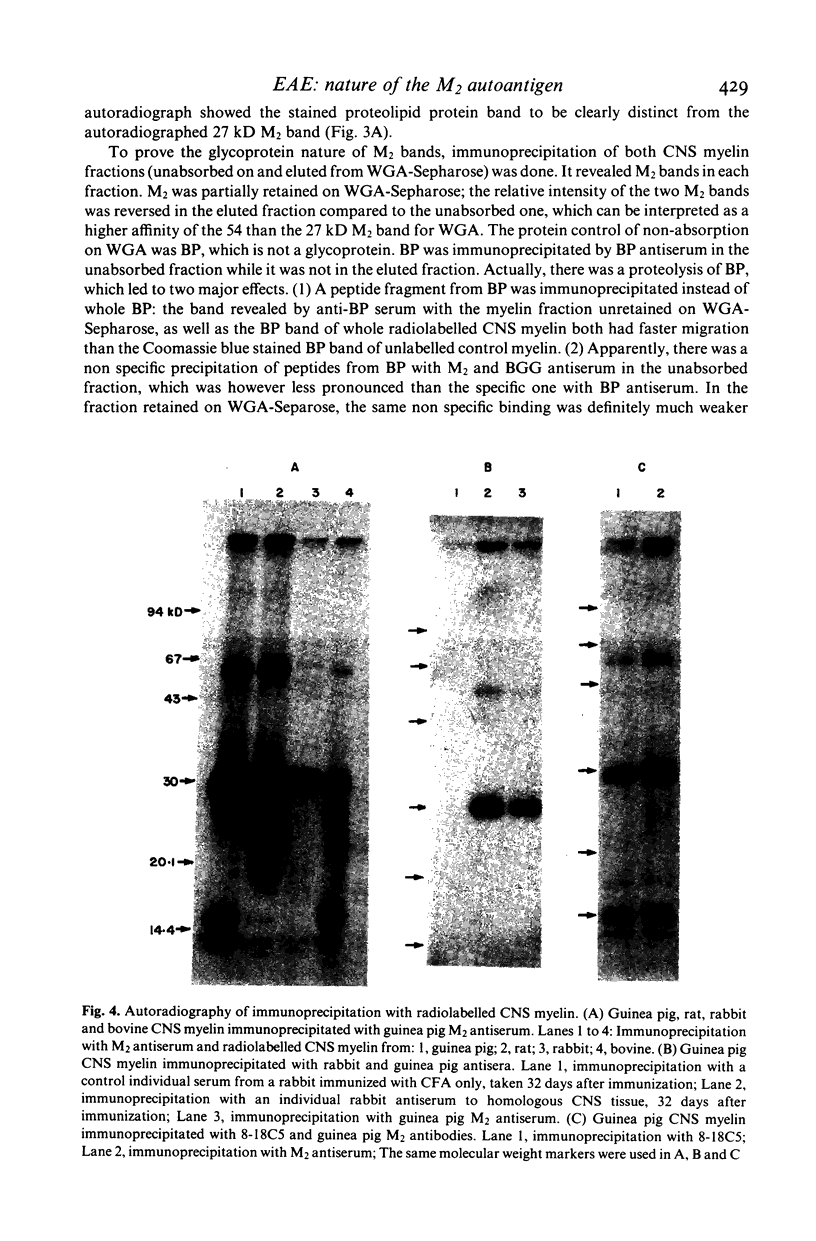
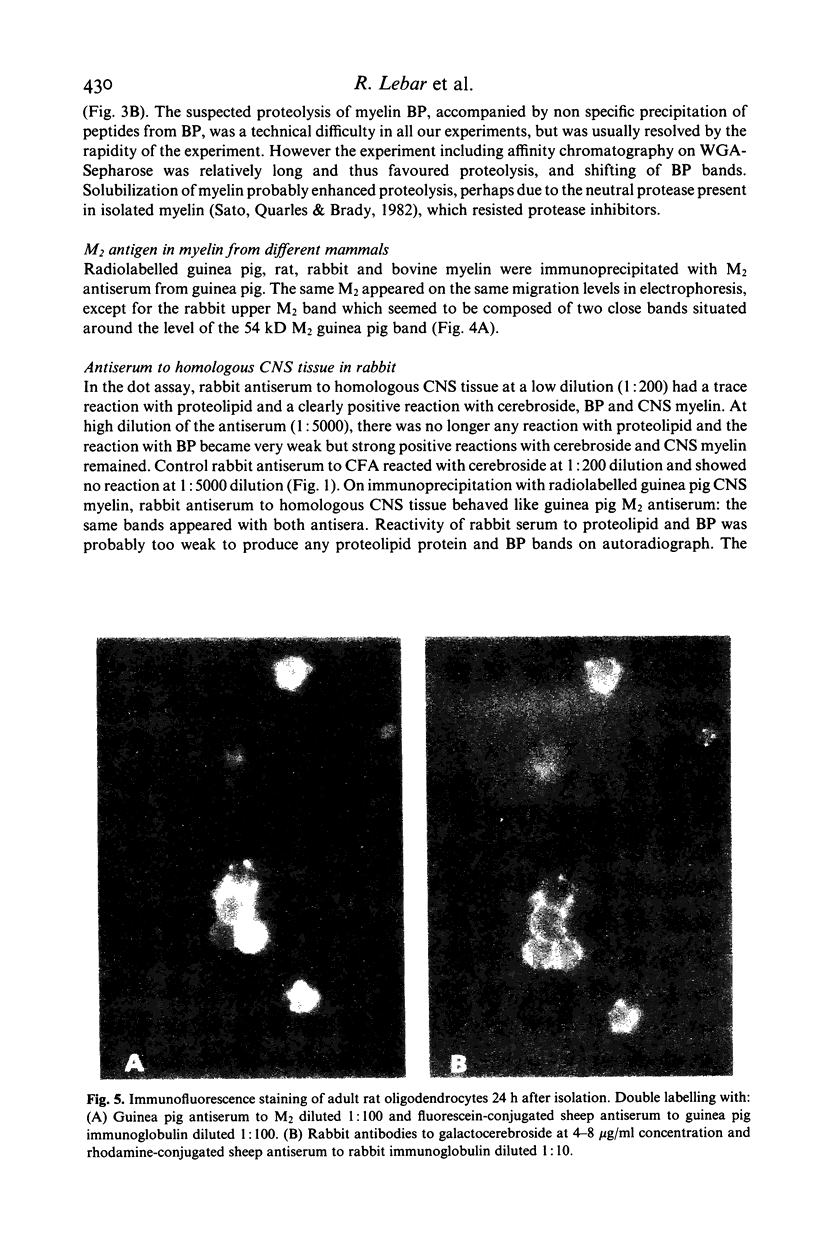




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agrawal H. C., Schmidt R. E., Agrawal D. In vivo incorporation of [3H]palmitic acid into PO protein, the major intrinsic protein of rat sciatic nerve myelin. J Biol Chem. 1983 May 25;258(10):6556–6560. [PubMed] [Google Scholar]
- Fritz R. B., Chou C. H., McFarlin D. E. Relapsing murine experimental allergic encephalomyelitis induced by myelin basic protein. J Immunol. 1983 Mar;130(3):1024–1026. [PubMed] [Google Scholar]
- Fry J. M., Weissbarth S., Lehrer G. M., Bornstein M. B. Cerebroside antibody inhibits sulfatide synthesis and myelination and demyelinates in cord tissue cultures. Science. 1974 Feb 8;183(4124):540–542. doi: 10.1126/science.183.4124.540. [DOI] [PubMed] [Google Scholar]
- Grundke-Iqbal I., Bornstein M. B. Multiple sclerosis: serum gamma globulin and demyelination in organ culture. Neurology. 1980 Jul;30(7 Pt 1):749–754. doi: 10.1212/wnl.30.7.749. [DOI] [PubMed] [Google Scholar]
- Hauw J. J., Boutry J. M., Crosnier-Suttin N., Robineaux R. Morphology of cultured guinea-pig cerebellum. I. Pattern of development. Comparison of phase contrast cinematography and silver impregnations of various cell types. Cell Tissue Res. 1974;152(2):141–164. doi: 10.1007/BF00224691. [DOI] [PubMed] [Google Scholar]
- Hauw J. J., Novikoff A. B., Novikoff P. M., Boutry J. M., Robineaux R. Culture of nervous tissue on collagen in Leighton tubes. Brain Res. 1972 Feb 25;37(2):301–309. doi: 10.1016/0006-8993(72)90675-0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lebar R., Boutry J. M., Vincent C., Robineaux R., Voisin G. A. Studies on autoimmune encephalomyelitis in the guinea pig. II. An in vitro investigation on the nature, properties, and specificity of the serum-demyelinating factor. J Immunol. 1976 May;116(5):1439–1446. [PubMed] [Google Scholar]
- Lebar R., Lees M. B. Dot immunobinding as a tool for the study of the CNS myelin antigen, M2. J Neuroimmunol. 1985 Apr;8(1):43–55. doi: 10.1016/s0165-5728(85)80046-1. [DOI] [PubMed] [Google Scholar]
- Lebar R., Vincent C., Fischer-le Boubennec E. Studies on autoimmune encephalomyelitis in the guinea pig--III. A comparative study of two autoantigens of central nervous system myelin. J Neurochem. 1979 May;32(5):1451–1460. doi: 10.1111/j.1471-4159.1979.tb11084.x. [DOI] [PubMed] [Google Scholar]
- Lebar R., Vincent C. Tentative identification of a second central nervous system myelin membrane autoantigen (M2) by a biochemical comparison with the basic protein (BP). J Neuroimmunol. 1981 Dec;1(4):367–389. doi: 10.1016/0165-5728(81)90018-7. [DOI] [PubMed] [Google Scholar]
- Lees M. B., Paxman S. Modification of the Lowry procedure for the analysis of proteolipid protein. Anal Biochem. 1972 May;47(1):184–192. doi: 10.1016/0003-2697(72)90291-6. [DOI] [PubMed] [Google Scholar]
- Linnington C., Webb M., Woodhams P. L. A novel myelin-associated glycoprotein defined by a mouse monoclonal antibody. J Neuroimmunol. 1984 Sep-Oct;6(6):387–396. doi: 10.1016/0165-5728(84)90064-x. [DOI] [PubMed] [Google Scholar]
- Lisak R. P., Pleasure D. E., Silberberg D. H., Manning M. C., Saida T. Long term culture of bovine oligodendroglia isolated with a Percoll gradient. Brain Res. 1981 Oct 26;223(1):107–122. doi: 10.1016/0006-8993(81)90809-x. [DOI] [PubMed] [Google Scholar]
- Moore G. R., Traugott U., Raine C. S. Survival of oligodendrocytes in chronic relapsing experimental autoimmune encephalomyelitis. J Neurol Sci. 1984 Aug;65(2):137–145. doi: 10.1016/0022-510x(84)90078-9. [DOI] [PubMed] [Google Scholar]
- Nicot C., Le T. N., Leprêtre M., Alfsen A. Study of Folch-Pi apoprotein. I. Isolation of two components, aggregation during delipidation. Biochim Biophys Acta. 1973 Sep 21;322(1):109–123. doi: 10.1016/0005-2795(73)90181-5. [DOI] [PubMed] [Google Scholar]
- Poduslo J. F., Harman J. L., McFarlin D. E. Lectin receptors in central nervous system myelin. J Neurochem. 1980 Jun;34(6):1733–1744. doi: 10.1111/j.1471-4159.1980.tb11268.x. [DOI] [PubMed] [Google Scholar]
- Poduslo J. F., Rodbard D. Molecular weight estimation using sodium dodecyl sulfate--pore gradient electrophoresis. Anal Biochem. 1980 Jan 15;101(2):394–406. doi: 10.1016/0003-2697(80)90205-5. [DOI] [PubMed] [Google Scholar]
- Raff M. C., Mirsky R., Fields K. L., Lisak R. P., Dorfman S. H., Silberberg D. H., Gregson N. A., Leibowitz S., Kennedy M. C. Galactocerebroside is a specific cell-surface antigenic marker for oligodendrocytes in culture. Nature. 1978 Aug 24;274(5673):813–816. [PubMed] [Google Scholar]
- Raine C. S., Johnson A. B., Marcus D. M., Suzuki A., Bornstein M. B. Demyelination in vitro. Absorption studies demonstrate that galactocerebroside is a major target. J Neurol Sci. 1981 Oct;52(1):117–131. doi: 10.1016/0022-510x(81)90140-4. [DOI] [PubMed] [Google Scholar]
- Reisner A. H., Nemes P., Bucholtz C. The use of Coomassie Brilliant Blue G250 perchloric acid solution for staining in electrophoresis and isoelectric focusing on polyacrylamide gels. Anal Biochem. 1975 Apr;64(2):509–516. doi: 10.1016/0003-2697(75)90461-3. [DOI] [PubMed] [Google Scholar]
- Reiss D. S., Lees M. B., Sapirstein V. S. Is Na + ATPase a myelin-associated enzyme? J Neurochem. 1981 Apr;36(4):1418–1426. doi: 10.1111/j.1471-4159.1981.tb00581.x. [DOI] [PubMed] [Google Scholar]
- Sato S., Quarles R. H., Brady R. O. Susceptibility of the myelin-associated glycoprotein and basic protein to a neutral protease in highly purified myelin from human and rat brain. J Neurochem. 1982 Jul;39(1):97–105. doi: 10.1111/j.1471-4159.1982.tb04706.x. [DOI] [PubMed] [Google Scholar]
- Schwerer B., Kitz K., Lassmann H., Bernheimer H. Serum antibodies against glycosphingolipids in chronic relapsing experimental allergic encephalomyelitis. Demonstration by ELISA and relation to serum in vivo demyelinating activity. J Neuroimmunol. 1984 Dec;7(2-3):107–119. doi: 10.1016/s0165-5728(84)80011-9. [DOI] [PubMed] [Google Scholar]
- Seil F. J., Falk G. A., Kies M. W., Alvord E. C., Jr The in vitro demyelinating activity of sera from guinea pigs sensitized with whole CNS and with purified encephalitogen. Exp Neurol. 1968 Dec;22(4):545–555. doi: 10.1016/0014-4886(68)90148-9. [DOI] [PubMed] [Google Scholar]
- Shepherd J., Bedford D. K., Morgan H. G. Radioiodination of human low density lipoprotein: a comparison of four methods. Clin Chim Acta. 1976 Jan 2;66(1):97–109. doi: 10.1016/0009-8981(76)90376-4. [DOI] [PubMed] [Google Scholar]
- Tabira T., Endoh M. Humoral immune responses to myelin basic protein, cerebroside and ganglioside in chronic relapsing experimental allergic encephalomyelitis of the guinea pig. J Neurol Sci. 1985 Feb;67(2):201–212. doi: 10.1016/0022-510x(85)90116-9. [DOI] [PubMed] [Google Scholar]