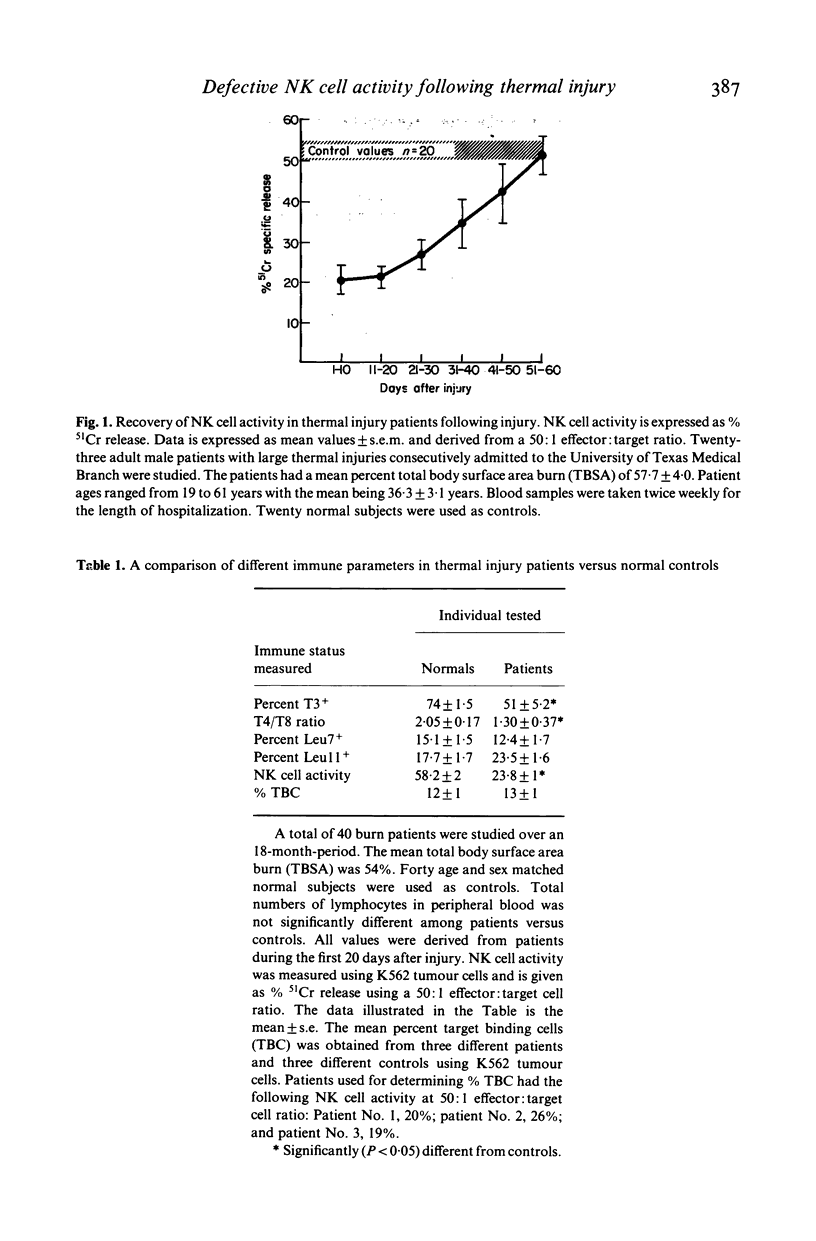
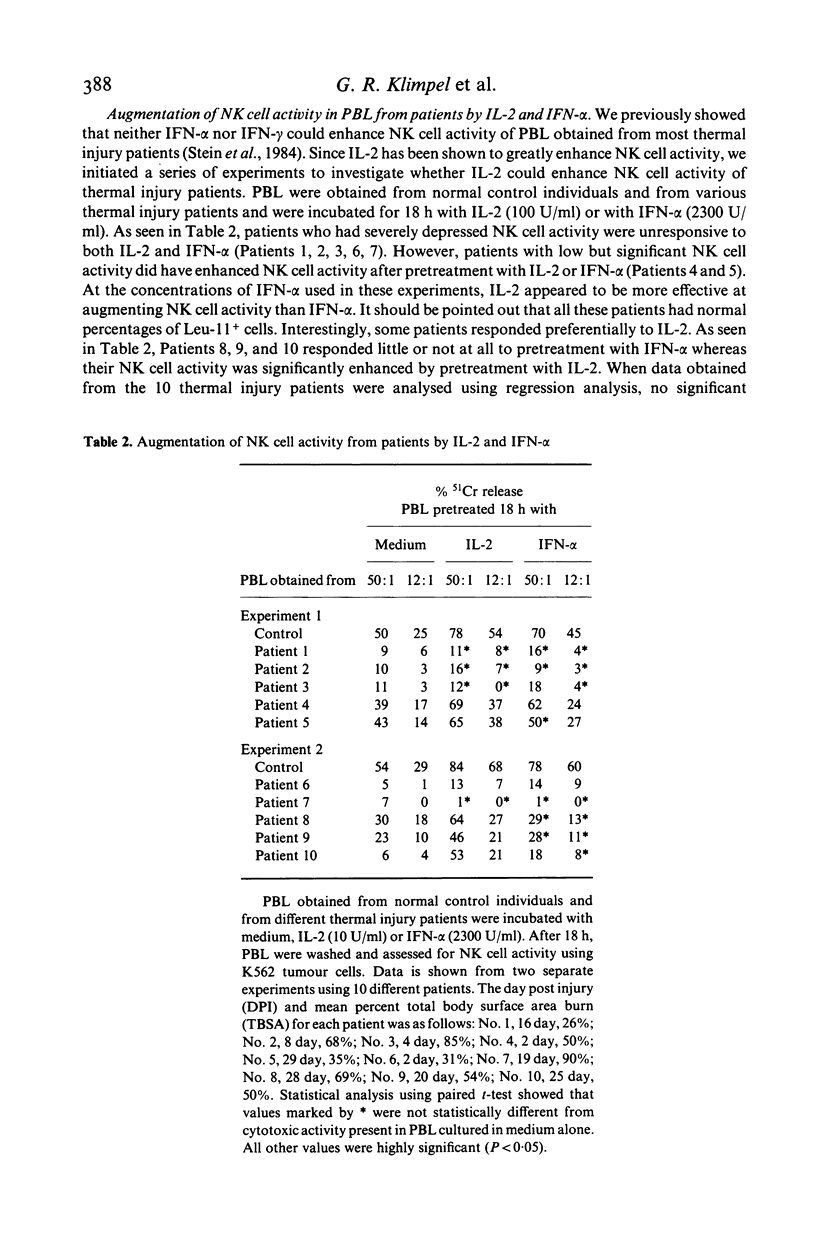
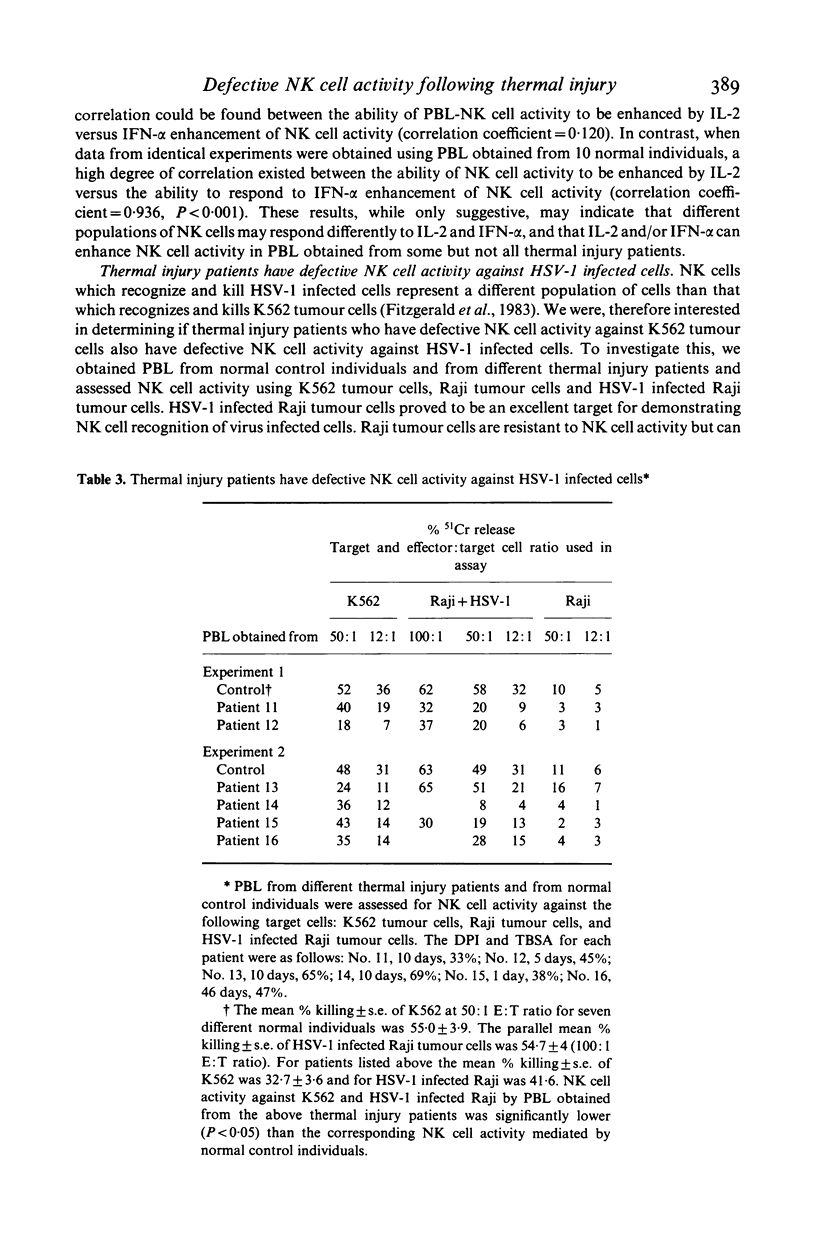
Abstract
Peripheral blood mononuclear lymphocytes (PBL) from thermal injury patients were examined for their ability to mediate natural killer (NK) cell activity against K562 tumour cells and against herpes simplex virus type 1 (HSV-1) infected Raji tumour cells. Using fluorescein isothiocyanate-conjugated monoclonal antibodies, the number of T3, T4, T8, Leu11, and Leu7 positive cells in PBL obtained from patients and normal controls was determined. Thermal injury patients had decreased levels of T3+ cells and a T4:T8 ratio which was significantly lower than that found in normal control individuals. Although patients had normal percentages of Leu7+ and Leu11+ cells, they had depressed NK cell activity against both K562 tumour cells and HSV-1 infected Raji cells. NK cell activity against K562 tumour cells was severely depressed during the first 20 days after injury. This defective NK cell activity did not appear to be due to a defect in PBL binding to the K562 tumour cells. In patients, the level of NK cell activity against HSV-1 infected cells did not correlate with the level of NK cell activity against K562 tumour cells. This finding further supports previous reports showing that NK cells which kill K562 tumour cells are different from the NK cell population which kills HSV-1 infected cells. Pretreatment of PBL obtained from patients with IL-2 or IFN-alpha, in some cases greatly enhanced NK cell killing of K562 tumour cells. However, IL-2 or IFN-alpha did not enhance NK cell activity in patients who had severely depressed levels of NK cell activity. Interestingly, in some patients, differential responsiveness to IL-2 and IFN-alpha was observed. In some patients, NK cell activity was enhanced by IL-2 but not by IFN-alpha. These results, while only suggestive, may indicate that different populations of NK cells respond preferentially to IL-2 and that IFN-alpha and/or IL-2 enhance NK cell activity in PBL obtained from some, but not all, thermal injury patients. Finally, this study clearly shows that thermal injury patients have defective NK cell activity not only against K562 tumour cells but also against virus-infected cells.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allavena P., Introna M., Mangioni C., Mantovani A. Inhibition of natural killer activity by tumor-associated lymphoid cells from ascites ovarian carcinomas. J Natl Cancer Inst. 1981 Aug;67(2):319–325. [PubMed] [Google Scholar]
- Auer I. O., Ziemer E., Sommer H. Immune status in Crohn's disease. V. Decreased in vitro natural killer cell activity in peripheral blood. Clin Exp Immunol. 1980 Oct;42(1):41–49. [PMC free article] [PubMed] [Google Scholar]
- Baker C. C., Miller C. L., Trunkey D. D. Predicting fatal sepsis in burn patients. J Trauma. 1979 Sep;19(9):641–648. doi: 10.1097/00005373-197909000-00001. [DOI] [PubMed] [Google Scholar]
- Fitzgerald P. A., Evans R., Kirkpatrick D., Lopez C. Heterogeneity of human NK cells: comparison of effectors that lyse HSV-1-infected fibroblasts and K562 erythroleukemia targets. J Immunol. 1983 Apr;130(4):1663–1667. [PubMed] [Google Scholar]
- Foley F. D., Greenawald K. A., Nash G., Pruitt B. A., Jr Herpesvirus infection in burned patients. N Engl J Med. 1970 Mar 19;282(12):652–656. doi: 10.1056/NEJM197003192821205. [DOI] [PubMed] [Google Scholar]
- Goto M., Tanimoto K., Chihara T., Horiuchi Y. Natural cell-mediated cytotoxicity in Sjögren's syndrome and rheumatoid arthritis. Arthritis Rheum. 1981 Nov;24(11):1377–1382. doi: 10.1002/art.1780241107. [DOI] [PubMed] [Google Scholar]
- Haliotis T., Roder J., Klein M., Ortaldo J., Fauci A. S., Herberman R. B. Chédiak-Higashi gene in humans I. Impairment of natural-killer function. J Exp Med. 1980 May 1;151(5):1039–1048. doi: 10.1084/jem.151.5.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herberman R. B., Ortaldo J. R. Natural killer cells: their roles in defenses against disease. Science. 1981 Oct 2;214(4516):24–30. doi: 10.1126/science.7025208. [DOI] [PubMed] [Google Scholar]
- Hersey P., Edwards A., McCarthy W. H. Tumour-related changes in natural killer cell activity in melanoma patients. Influence of stage of disease, tumour thickness and age of patients. Int J Cancer. 1980 Feb 15;25(2):187–194. doi: 10.1002/ijc.2910250204. [DOI] [PubMed] [Google Scholar]
- Hoffman T. Natural killer funciton in systemic lupus erythematosus. Arthritis Rheum. 1980 Jan;23(1):30–35. doi: 10.1002/art.1780230106. [DOI] [PubMed] [Google Scholar]
- Katz P., Zaytoun A. M., Lee J. H., Jr, Panush R. S., Longley S. Abnormal natural killer cell activity in systemic lupus erythematosus: an intrinsic defect in the lytic event. J Immunol. 1982 Nov;129(5):1966–1971. [PubMed] [Google Scholar]
- Klimpel G. R., Dorshkind K., Klimpel K. D., Rosse C. Bone marrow cytotoxic precursor T cells: alloantigen-induced cytotoxic T-cell responses by murine bone marrow cells in vitro. Cell Immunol. 1981 Jun;61(1):154–164. doi: 10.1016/0008-8749(81)90362-2. [DOI] [PubMed] [Google Scholar]
- Klimpel G. R., Niesel D. W., Klimpel K. D. Natural cytotoxic effector cell activity against Shigella flexneri-infected HeLa cells. J Immunol. 1986 Feb 1;136(3):1081–1086. [PubMed] [Google Scholar]
- Kohl S., Ericsson C. D. Cellular cytotoxicity to herpes simplex virus-infected cells of leukocytes from patients with serious burns. Clin Immunol Immunopathol. 1982 Aug;24(2):171–178. doi: 10.1016/0090-1229(82)90228-8. [DOI] [PubMed] [Google Scholar]
- Linnemann C. C., Jr, MacMillan B. G. Viral infections in pediatric burn patients. Am J Dis Child. 1981 Aug;135(8):750–753. doi: 10.1001/archpedi.1981.02130320064021. [DOI] [PubMed] [Google Scholar]
- Miller C. L., Baker C. C. Changes in lymphocyte activity after thermal injury. The role of suppressor cells. J Clin Invest. 1979 Feb;63(2):202–210. doi: 10.1172/JCI109290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan D. R., DuPont H. L., Gonik B., Kohl S. Cytotoxicity of human peripheral blood and colostral leukocytes against Shigella species. Infect Immun. 1984 Oct;46(1):25–33. doi: 10.1128/iai.46.1.25-33.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nencioni L., Villa L., Boraschi D., Berti B., Tagliabue A. Natural and antibody-dependent cell-mediated activity against Salmonella typhimurium by peripheral and intestinal lymphoid cells in mice. J Immunol. 1983 Feb;130(2):903–907. [PubMed] [Google Scholar]
- Ninnemann J. L., Fisher J. C., Frank H. A. Prolonged survival of human skin allografts following thermal injury. Transplantation. 1978 Feb;25(2):69–72. doi: 10.1097/00007890-197802000-00006. [DOI] [PubMed] [Google Scholar]
- Seeman J., Konigová R. Cytomegalovirus infection in severely burned patients. Acta Chir Plast. 1976;18(3):142–151. [PubMed] [Google Scholar]
- Seeman J., Königová R., Lysenková I. Fatal outcome of cytomegalovirus infections in severe burns. Acta Chir Plast. 1980;22(3):166–170. [PubMed] [Google Scholar]
- Speelman D., Li J. L., Ramanujam V. M., Legator M. S., Albrecht T. Herpes virus inactivation by chemical carcinogens: differential inactivation of herpes simplex viruses by 4-nitroquinoline 1-oxide and related compounds. Environ Mutagen. 1981;3(4):467–476. doi: 10.1002/em.2860030407. [DOI] [PubMed] [Google Scholar]
- Stein M. D., Gamble D. N., Klimpel K. D., Herndon D. N., Klimpel G. R. Natural killer cell defects resulting from thermal injury. Cell Immunol. 1984 Jul;86(2):551–556. doi: 10.1016/0008-8749(84)90412-x. [DOI] [PubMed] [Google Scholar]
- Steinhauer E. H., Doyle A. T., Reed J., Kadish A. S. Defective natural cytotoxicity in patients with cancer: normal number of effector cells but decreased recycling capacity in patients with advanced disease. J Immunol. 1982 Nov;129(5):2255–2259. [PubMed] [Google Scholar]
- Trinchieri G., Perussia B. Human natural killer cells: biologic and pathologic aspects. Lab Invest. 1984 May;50(5):489–513. [PubMed] [Google Scholar]
- Wolfe J. H., Wu A. V., O'Connor N. E., Saporoschetz I., Mannick J. A. Anergy, immunosuppressive serum, and impaired lymphocyte blastogenesis in burn patients. Arch Surg. 1982 Oct;117(10):1266–1271. doi: 10.1001/archsurg.1982.01380340002002. [DOI] [PubMed] [Google Scholar]
- Wood J. J., Rodrick M. L., O'Mahony J. B., Palder S. B., Saporoschetz I., D'Eon P., Mannick J. A. Inadequate interleukin 2 production. A fundamental immunological deficiency in patients with major burns. Ann Surg. 1984 Sep;200(3):311–320. doi: 10.1097/00000658-198409000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler H. W., Kay N. E., Zarling J. M. Deficiency of natural killer cell activity in patients with chronic lymphocytic leukemia. Int J Cancer. 1981 Mar 15;27(3):321–327. doi: 10.1002/ijc.2910270310. [DOI] [PubMed] [Google Scholar]


