Abstract
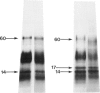
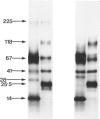
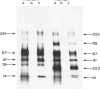
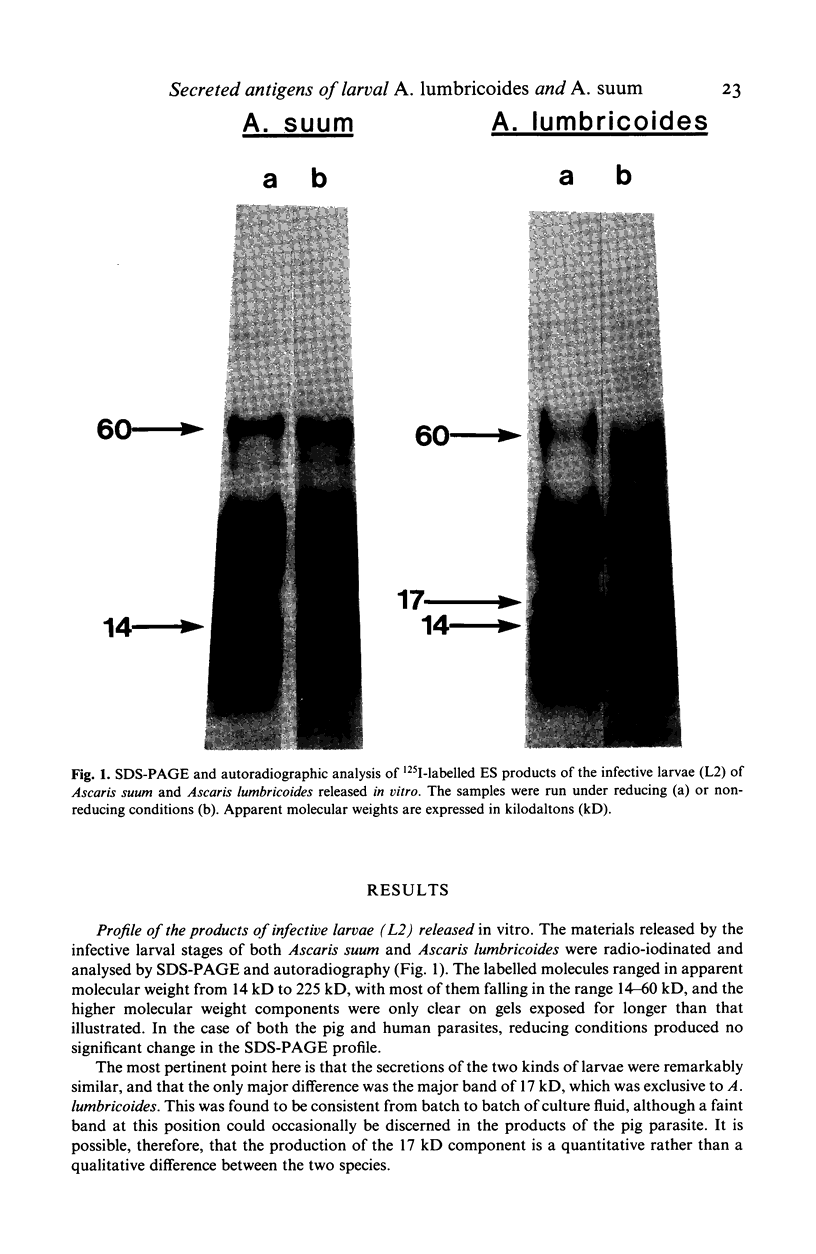
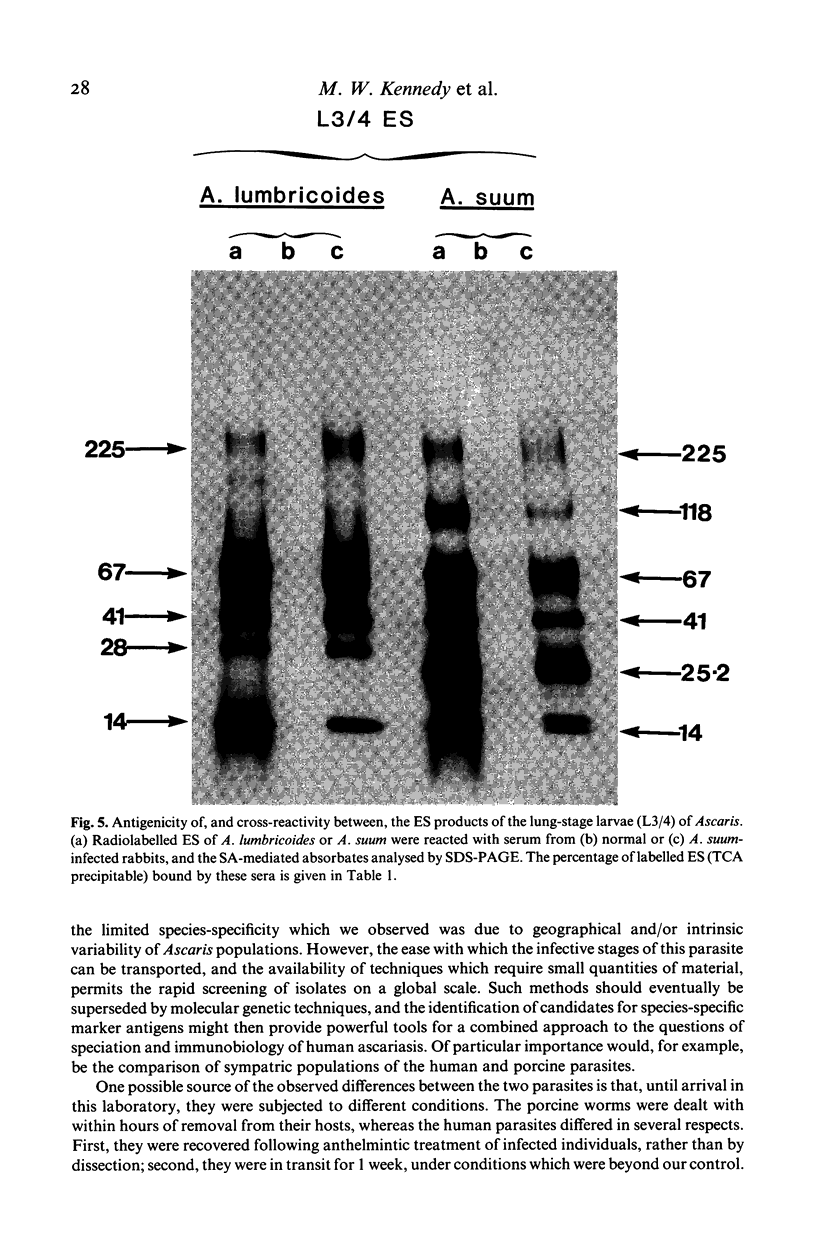
The materials released in vitro by the tissue-parasitic larval stages of the large roundworm of man, Ascaris lumbricoides, were analysed by radio-iodination, immunoprecipitation, and SDS-PAGE. The antigens were found to be heterogeneous, ranging in molecular weight from 14 to 410 kD, and were found to alter radically during the parasites' migration to the lungs. The antigens secreted by the infective and lung-stage larvae of the pig homologue, Ascaris suum, were compared with those of the human worms. This revealed a remarkable degree of homology between the products of the two, at both the molecular and immunological levels. The two species could be discriminated, however, on the basis of the SDS-PAGE profiles of the antigens secreted by both developmental stages of the parasites examined. Finally, antiserum to the canine ascarid infective to man, Toxocara canis, was found to precipitate a significant proportion of Ascaris-secreted molecules. These studies, therefore, confirm the potent antigenicity of excretory/secretory materials, and their potential for use in immunodiagnosis, but predict serious difficulties for seroepidemiology and the specific detection of ascariasis in man.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Crompton D. W. Chronic ascariasis and malnutrition. Parasitol Today. 1985 Aug;1(2):47–52. doi: 10.1016/0169-4758(85)90114-0. [DOI] [PubMed] [Google Scholar]
- Kennedy M. W., Qureshi F. Stage-specific secreted antigens of the parasitic larval stages of the nematode Ascaris. Immunology. 1986 Jul;58(3):515–522. [PMC free article] [PubMed] [Google Scholar]
- Kessler S. W. Rapid isolation of antigens from cells with a staphylococcal protein A-antibody adsorbent: parameters of the interaction of antibody-antigen complexes with protein A. J Immunol. 1975 Dec;115(6):1617–1624. [PubMed] [Google Scholar]
- Maizels R. M., Meghji M., Ogilvie B. M. Restricted sets of parasite antigens from the surface of different stages and sexes of the nematode parasite Nippostrongylus brasiliensis. Immunology. 1983 Jan;48(1):107–121. [PMC free article] [PubMed] [Google Scholar]
- Maizels R. M., de Savigny D., Ogilvie B. M. Characterization of surface and excretory-secretory antigens of Toxocara canis infective larvae. Parasite Immunol. 1984 Jan;6(1):23–37. doi: 10.1111/j.1365-3024.1984.tb00779.x. [DOI] [PubMed] [Google Scholar]
- Markwell M. A., Fox C. F. Surface-specific iodination of membrane proteins of viruses and eucaryotic cells using 1,3,4,6-tetrachloro-3alpha,6alpha-diphenylglycoluril. Biochemistry. 1978 Oct 31;17(22):4807–4817. doi: 10.1021/bi00615a031. [DOI] [PubMed] [Google Scholar]
- O'Donnell I. J., Mitchell G. F. An investigation of the allergens of Ascaris lumbricoides using a radioallergosorbent test (RAST) and sera of naturally infected humans: comparison with an allergen for mice identified by a passive cutaneous anaphylaxis test. Aust J Biol Sci. 1978 Oct;31(5):459–487. doi: 10.1071/bi9780459. [DOI] [PubMed] [Google Scholar]
- O'Donnell I. J., Mitchell G. F. An investigation of the antigens of Ascaris lumbricoides using a radioimmunoassay and sera of naturally infected humans. Int Arch Allergy Appl Immunol. 1980;61(2):213–219. doi: 10.1159/000232435. [DOI] [PubMed] [Google Scholar]
- SPRENT J. F. A. Anatomical distinction between human and pig strains of Ascaris. Nature. 1952 Oct 11;170(4328):627–628. doi: 10.1038/170627b0. [DOI] [PubMed] [Google Scholar]
- Stromberg B. E. IgE and IgG1 antibody production by a soluble product of Ascaris suum in the guinea-pig. Immunology. 1979 Nov;38(3):489–495. [PMC free article] [PubMed] [Google Scholar]
- Stromberg B. E. Potentiation of the reaginic (IgE) antibody response to ovalbumin in the guinea pig with a soluble metabolic product from Ascaris suum. J Immunol. 1980 Aug;125(2):833–836. [PubMed] [Google Scholar]
- Stromberg B. E. The isolation and partial characterization of a protective antigen from developing larvae of Ascaris suum. Int J Parasitol. 1979 Aug;9(4):307–311. doi: 10.1016/0020-7519(79)90079-1. [DOI] [PubMed] [Google Scholar]
- TAFFS L. F., VOLLER A. IN VITRO FLUORESCENT ANTIBODY STUDIES ON ASCARIS LUMBRICOIDES AND ASCARIS SUUM. Trans R Soc Trop Med Hyg. 1963 Sep;57:353–358. doi: 10.1016/0035-9203(63)90098-1. [DOI] [PubMed] [Google Scholar]
- Tsuji M., Hayashi T., Yamamoto S., Sakata Y., Toshida T. IgE-type antibodies to Ascaris antigens in man. Int Arch Allergy Appl Immunol. 1977;55(1-6):78–81. doi: 10.1159/000231912. [DOI] [PubMed] [Google Scholar]
- Urban J. F., Jr, Douvres F. W., Xu S. Culture requirements of Ascaris suum larvae using a stationary multi-well system: increased survival, development and growth with cholesterol. Vet Parasitol. 1984 Jan;14(1):33–42. doi: 10.1016/0304-4017(84)90131-6. [DOI] [PubMed] [Google Scholar]
- Urban J. F., Jr, Romanowski R. D. Ascaris suum: protective immunity in pigs immunized with products from eggs and larvae. Exp Parasitol. 1985 Oct;60(2):245–254. doi: 10.1016/0014-4894(85)90028-1. [DOI] [PubMed] [Google Scholar]
- Voller A., De Savigny D. Diagnostic serology of tropical parasitic diseases. J Immunol Methods. 1981;46(1):1–29. doi: 10.1016/0022-1759(81)90328-8. [DOI] [PubMed] [Google Scholar]
- Welch J. S., Dobson C., Chopra S. Immunodiagnosis of Entamoeba histolytica and Ascaris lumbricoides infections in Caucasian and aboriginal Australians. Trans R Soc Trop Med Hyg. 1986;80(2):240–247. doi: 10.1016/0035-9203(86)90024-6. [DOI] [PubMed] [Google Scholar]