Abstract
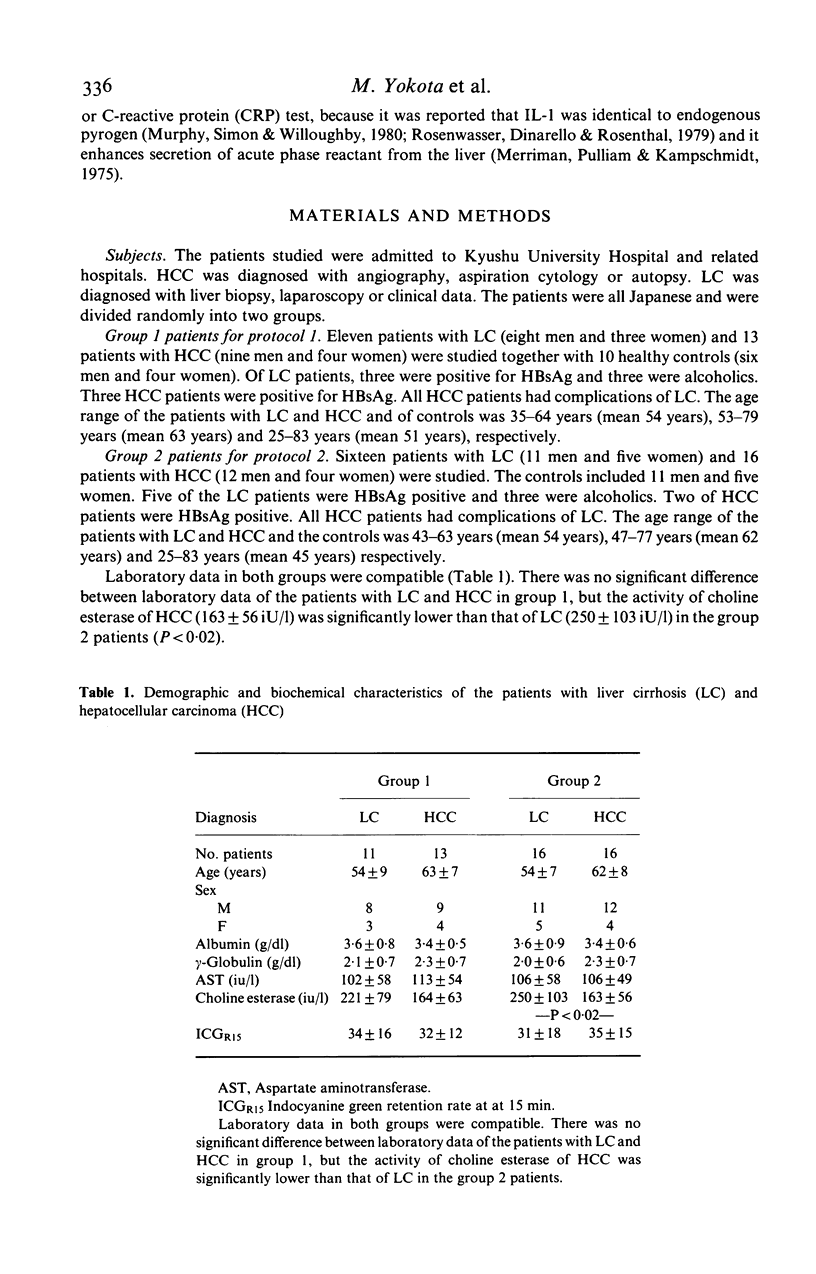
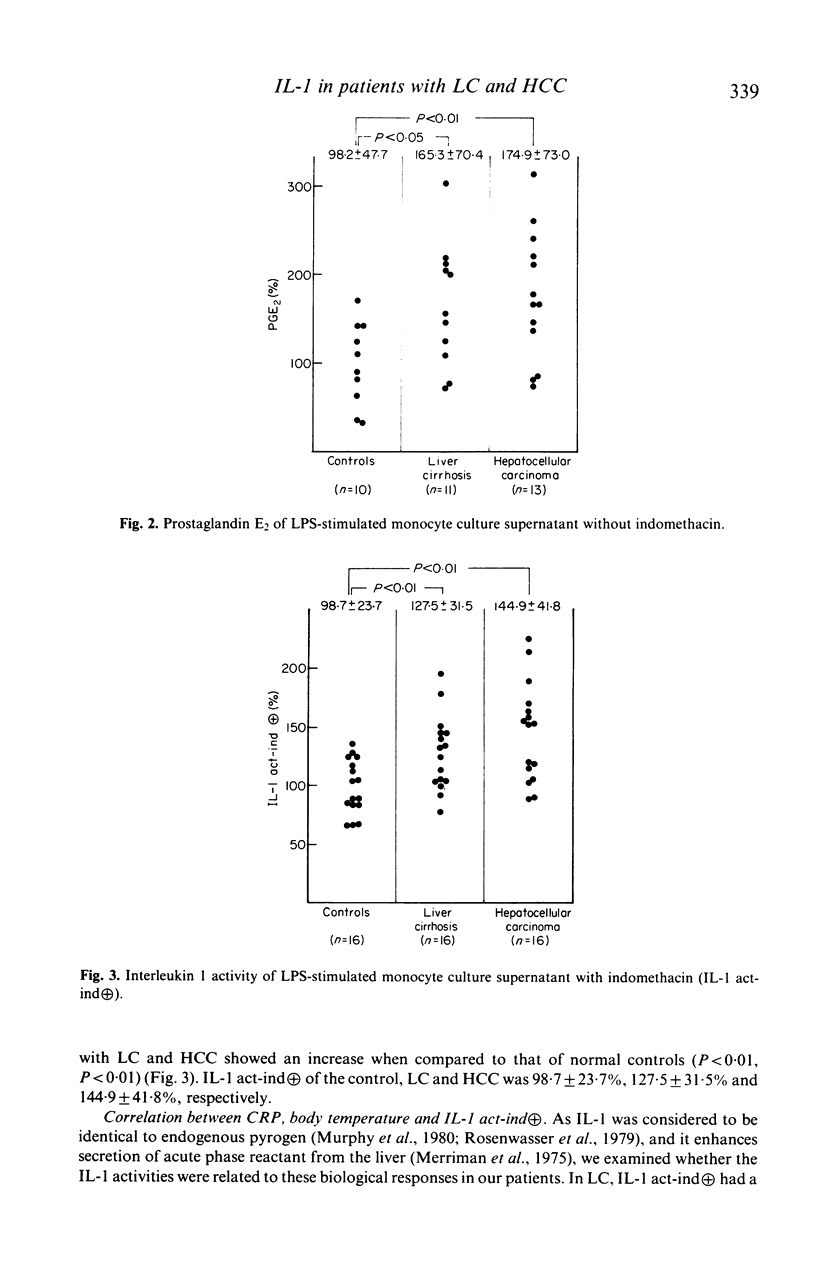
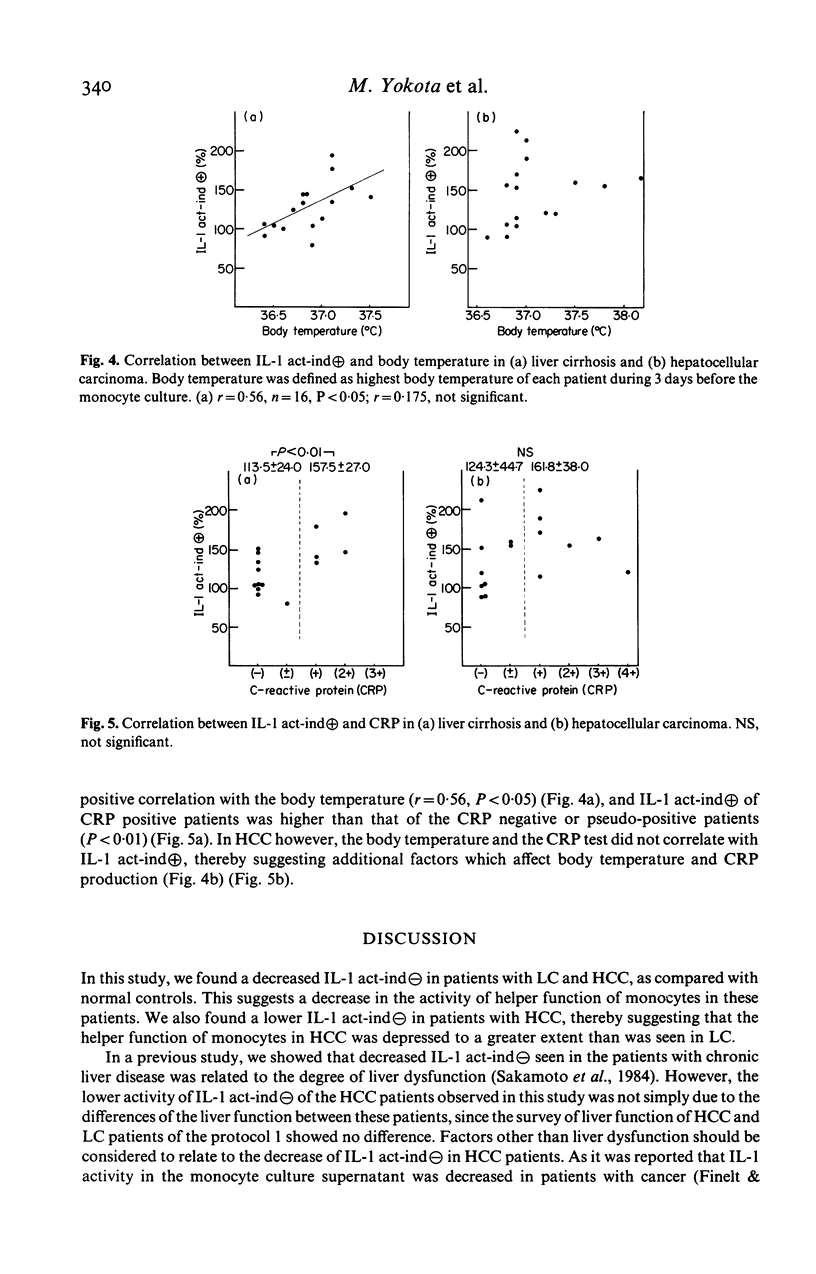
Immunoregulatory function of peripheral blood monocytes was studied in patients with hepatocellular carcinoma (HCC) and liver cirrhosis (LC), by assaying interleukin 1 (IL-1) and prostaglandin E2 (PGE2) in the culture supernatant of lipopolysaccharide-stimulated monocytes. IL-1 activity of the monocyte culture supernatant without indomethacin was decreased in patients with HCC and LC, compared with that of controls. The activity was lower in patients with HCC than that in those with LC. The PGE2 content of the culture supernatant of monocytes from patients with LC and HCC was increased, compared to normal controls. To avoid the effect of PGE2 on the IL-1 assay, we cultured the monocytes with addition of indomethacin and assayed IL-1 activity in the culture supernatant. As a result, monocyte IL-1 production was increased in patients with HCC and LC, compared with normal controls. The decrease in IL-1 activity of the supernatant without indomethacin of patients with LC and HCC was considered to be due to increased secretion of PGE2 by the monocytes. Therefore, monocytes from patients with HCC and LC had an increased capacity of secreting both IL-1 and PGE2 over normal controls, but the effect of the suppressor function (PGE2 secretion) dominated in these patients.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDERSON H. C., McCARTY M. Determination of C-reactive protein in the blood as a measure of the activity of the disease process in acute rheumatic fever. Am J Med. 1950 Apr;8(4):445–455. doi: 10.1016/0002-9343(50)90226-9. [DOI] [PubMed] [Google Scholar]
- Bonney R. J., Wightman P. D., Davies P., Sadowski S. J., Kuehl F. A., Jr, Humes J. L. Regulation of prostaglandin synthesis and of the selective release of lysosomal hydrolases by mouse peritoneal macrophages. Biochem J. 1978 Nov 15;176(2):433–442. doi: 10.1042/bj1760433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunda M. J., Herberman R. B., Holden H. T. Inhibition of murine natural killer cell activity by prostaglandins. J Immunol. 1980 Jun;124(6):2682–2687. [PubMed] [Google Scholar]
- Dinarello C. A., Bernheim H. A., Duff G. W., Le H. V., Nagabhushan T. L., Hamilton N. C., Coceani F. Mechanisms of fever induced by recombinant human interferon. J Clin Invest. 1984 Sep;74(3):906–913. doi: 10.1172/JCI111508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello C. A., Cannon J. G., Wolff S. M., Bernheim H. A., Beutler B., Cerami A., Figari I. S., Palladino M. A., Jr, O'Connor J. V. Tumor necrosis factor (cachectin) is an endogenous pyrogen and induces production of interleukin 1. J Exp Med. 1986 Jun 1;163(6):1433–1450. doi: 10.1084/jem.163.6.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello C. A. Interleukin-1 and the pathogenesis of the acute-phase response. N Engl J Med. 1984 Nov 29;311(22):1413–1418. doi: 10.1056/NEJM198411293112205. [DOI] [PubMed] [Google Scholar]
- Dinarello C. A., Marnoy S. O., Rosenwasser L. J. Role of arachidonate metabolism in the immunoregulatory function of human leukocytic pyrogen/lymphocyte-activating factor/interleukin 1. J Immunol. 1983 Feb;130(2):890–895. [PubMed] [Google Scholar]
- Finelt M., Hoffmann M. K. A human monocyte function test: release of B-cell differentiation factor (BDF). Clin Immunol Immunopathol. 1979 Mar;12(3):281–288. doi: 10.1016/0090-1229(79)90031-x. [DOI] [PubMed] [Google Scholar]
- Gery I., Waksman B. H. Potentiation of the T-lymphocyte response to mitogens. II. The cellular source of potentiating mediator(s). J Exp Med. 1972 Jul 1;136(1):143–155. doi: 10.1084/jem.136.1.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haller O., Hansson M., Kiessling R., Wigzell H. Role of non-conventional natural killer cells in resistance against syngeneic tumour cells in vivo. Nature. 1977 Dec 15;270(5638):609–611. doi: 10.1038/270609a0. [DOI] [PubMed] [Google Scholar]
- Hassner A., Kletter Y., Shlag D., Yedvab M., Aronson M., Shibolet S. Impaired monocyte function in liver cirrhosis. Br Med J (Clin Res Ed) 1981 Apr 18;282(6272):1262–1263. doi: 10.1136/bmj.282.6272.1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holdstock G., Chastenay B. F., Krawitt E. L. Studies on lymphocyte hyporesponsiveness in cirrhosis: the role of increased monocyte suppressor cell activity. Gastroenterology. 1982 Feb;82(2):206–212. [PubMed] [Google Scholar]
- Merriman C. R., Pulliam L. A., Kampschmidt R. F. Effect of leukocytic endogenous mediator on C-reactive protein in rabbits. Proc Soc Exp Biol Med. 1975 Jul;149(3):782–784. doi: 10.3181/00379727-149-38898. [DOI] [PubMed] [Google Scholar]
- Murphy P. A., Simon P. L., Willoughby W. F. Endogenous pyrogens made by rabbit peritoneal exudate cells are identical with lymphocyte-activating factors made by rabbit alveolar macrophages. J Immunol. 1980 May;124(5):2498–2501. [PubMed] [Google Scholar]
- Nakamura T., Morizane T., Watanabe T., Tsuchimoto K., Inagaki Y., Kumagai N., Tsuchiya M. Decreased natural killer activity in patients with liver cirrhosis. Int J Cancer. 1983 Nov 15;32(5):573–575. doi: 10.1002/ijc.2910320509. [DOI] [PubMed] [Google Scholar]
- Rosenwasser L. J., Dinarello C. A., Rosenthal A. S. Adherent cell function in murine T-lymphocyte antigen recognition. IV. Enhancement of murine T-cell antigen recognition by human leukocytic pyrogen. J Exp Med. 1979 Sep 19;150(3):709–714. doi: 10.1084/jem.150.3.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto S., Koga S., Ibayashi H. Interleukin 1 activity in culture supernatant of lipopolysaccharide-stimulated monocytes from patients with chronic liver disease. Hepatogastroenterology. 1984 Dec;31(6):248–253. [PubMed] [Google Scholar]
- Talmadge J. E., Meyers K. M., Prieur D. J., Starkey J. R. Role of NK cells in tumour growth and metastasis in beige mice. Nature. 1980 Apr 17;284(5757):622–624. doi: 10.1038/284622a0. [DOI] [PubMed] [Google Scholar]
- Wood D. D. Purification and properties of human B cell-activating factor. J Immunol. 1979 Nov;123(5):2395–2399. [PubMed] [Google Scholar]


