Abstract
We have previously identified an ecotype of the hyperaccumulator Thlaspi caerulescens (Ganges), which is far superior to other ecotypes (including Prayon) in Cd uptake. In this study, we investigated the effect of Fe status on the uptake of Cd and Zn in the Ganges and Prayon ecotypes, and the kinetics of Cd and Zn influx using radioisotopes. Furthermore, the T. caerulescens ZIP (Zn-regulated transporter/Fe-regulated transporter-like protein) genes TcZNT1-G and TcIRT1-G were cloned from the Ganges ecotype and their expression under Fe-sufficient and -deficient conditions was analyzed. Both short- and long-term studies revealed that Cd uptake was significantly enhanced by Fe deficiency only in the Ganges ecotype. The concentration-dependent kinetics of Cd influx showed that the Vmax of Cd was 3 times greater in Fe-deficient Ganges plants compared with Fe-sufficient plants. In Prayon, Fe deficiency did not induce a significant increase in Vmax for Cd. Zn uptake was not influenced by the Fe status of the plants in either of the ecotypes. These results are in agreement with the gene expression study. The abundance of ZNT1-G mRNA was similar between the Fe treatments and between the two ecotypes. In contrast, abundance of the TcIRT1-G mRNA was greatly increased only in Ganges root tissue under Fe-deficient conditions. The present results indicate that the stimulatory effect of Fe deficiency on Cd uptake in Ganges may be related to an up-regulation in the expression of genes encoding for Fe2+ uptake, possibly TcIRT1-G.
Hyperaccumulation of heavy metals by higher plants is a complex phenomenon. It is likely to involve several steps, including metal transport across plasma membranes of root cells, xylem loading and translocation, detoxification, and sequestration of metals at the whole plant and cellular level. Thlaspi caerulescens J & C Presl is a well-known hyperaccumulator of Zn, and has been the subject of numerous investigations aiming to understand the physiological and molecular mechanisms responsible for the accumulation of Zn. It was shown that in T. caerulescens, Zn is taken up via a high-affinity Zn transport system (Lasat et al., 1996). Genes encoding these transporters, ZNT1 and ZNT2, were recently cloned from T. caerulescens by Pence et al. (2000) and Assunção et al. (2001). Compared with the nonaccumulator Thlaspi arvense, ZNT1 was found to be expressed at much higher levels in both roots and shoots of T. caerulescens. Also, down-regulation of the ZNT1 expression occurred at a much higher concentration of Zn supply (50 μm) for T. caerulescens than for T. arvense (1 μm; Pence et al., 2000). The results suggest that an alteration in the regulation of Zn transport by Zn status plays a role in enhanced Zn uptake and Zn hyperaccumulation in T. caerulescens.
T. caerulescens is the only known species to hyperaccumulate Cd (defined as being capable of accumulating more than 100 mg Cd kg−1 in shoots) from geobotanical surveys (Baker et al., 2000). However, the mechanism of Cd uptake in this hyperaccumulator plant is still not completely understood. It is often assumed that Cd, and other heavy metals without a biological function, are taken up by transporters for essential elements because of a lack of specificity. There is evidence that ZNT1 can also mediate the transport of Cd with low affinity in T. caerulescens (Pence et al., 2000). It was assumed previously that a common mechanism was responsible for Zn and Cd hyperaccumulation in T. caerulescens (Baker et al., 1994). In nonaccumulator plants, Clemens et al. (1998) showed that a Ca transport pathway could be involved in the uptake of Cd, albeit with a low affinity. Members of the ZIP (Zn-regulated transporter/Fe-regulated transporter-like protein) and Nramp (natural resistance-associated macrophage protein) gene families have been shown to transport several divalent cations including Cd (Eide et al., 1996; Korshunova et al., 1999; Guerinot, 2000; Thomine et al., 2000). A Fe transporter cloned from Arabidopsis (IRT1) is induced by Fe deficiency in pea (Pisum sativum) and might facilitate the transport of Cd (Cohen et al., 1998). The effect of Fe status on Cd accumulation has not been studied in the hyperaccumulator T. caerulescens.
We recently identified an ecotype (named Ganges) of T. caerulescens from southern France, which is far superior in Cd accumulation to other populations tested, including the population from Prayon in Belgium (Lombi et al., 2000, 2001). Yet, the different populations showed a similar ability to hyperaccumulate Zn. Also, the Ganges ecotype is more tolerant to Cd than the Prayon ecotype, being able to accumulate up to 10,000 mg Cd kg−1 in the shoot dry matter without suffering phytotoxicity under hydroponic culture conditions. Kinetics studies revealed a marked difference in Cd influx, but little difference in Zn influx, between the Ganges and Prayon ecotypes (Lombi et al., 2001). The Ganges ecotype showed a clear saturable component in the low-Cd concentration range, but this was much less evident in the Prayon ecotype. The Km values were not significantly different between the two ecotypes, but the Vmax for Cd was 5-fold higher in the Ganges than in the Prayon ecotype. In addition, at an equimolar concentration, Zn inhibited Cd uptake by Prayon but not by Ganges. From the physiological evidence, we postulated that a transport system with a high affinity for Cd, and other than ZNT1, was highly expressed in the Ganges ecotype (Lombi et al., 2001).
The present paper investigates whether the Fe status plays a role in Cd uptake by the two contrasting ecotypes of T. caerulescens. We investigated the effect of Fe deficiency on the kinetics of Cd and Zn influx, and on long-term accumulation of Cd and Zn. Furthermore, we cloned homologs of the genes IRT1 and ZNT1 from T. caerulescens and examined their expression in the two ecotypes in response to Fe status.
RESULTS
Cd and Zn Accumulation and Growth in Response to Fe Status
The growth of both ecotypes of T. caerulescens was decreased significantly (P < 0.01) by Fe-deficient conditions (Fig. 1A). Compared with the zero Fe treatment, the shoot biomass of both ecotypes almost doubled when Fe-EDDHA was present in solution at a concentration of 60 μm. Plants grown in solution containing less than 15 μm Fe-EDDHA showed chlorosis. The biomass of Prayon plants was significantly higher than Ganges because of the initial larger seedlings of the former.
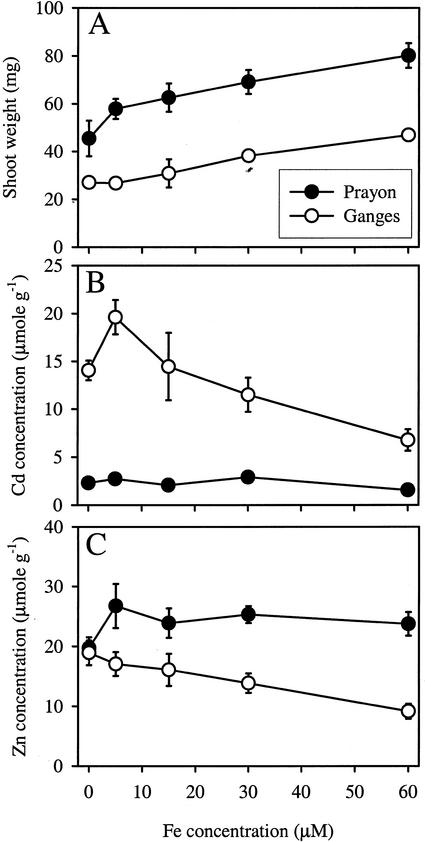
Figure 1.
Shoot weight (A) and accumulation in the shoots of Cd (B) and Zn (C) in two ecotypes of T. caerulescens grown for 10 d in hydroponics with a range of Fe-ethylenediamine-di(o-hydroxyphenylacetic acid) (EDDHA) concentrations. Values are means ± se, n = 4.
The concentration of Cd in the shoot was 4.4- to 7.2-fold higher in the Ganges ecotype than in the Prayon ecotype (P < 0.001; Fig. 1B). Despite smaller biomass in the Ganges ecotype, its total Cd uptake was 2.2- to 3.7-fold higher than the uptake by the Prayon ecotype. Cd concentration did not vary significantly in the Prayon ecotype with decreasing concentrations of Fe in solution. In contrast, the concentration of Cd in the Ganges ecotype increased by more than 2-fold when Fe in solution decreased from 60 to 5 μm. When no Fe was present in solution, the Cd concentration was lower than at 5 μm Fe-EDDHA. This was probably because of the extreme nutrient-deficient conditions in this treatment that may have impaired the uptake processes.
The concentration of Zn in the shoots of the two ecotypes followed an opposite pattern in comparison with Cd. In fact, the Zn concentration in Prayon was higher than in Ganges (Fig. 1C). Furthermore, the Zn concentration in the shoot of Prayon plants did not significantly change when Fe-EDDHA was supplied in the range 5 to 60 μm, whereas Zn concentration in the Ganges plants decreased by 52%.
The two ecotypes showed a significant (P < 0.001) difference in terms of the ratio between the molar concentrations of Cd and Zn in the shoots. In Ganges, this ratio approached 1, with an average value between the Fe treatments of 0.87, whereas in Prayon, the ratio was only 0.10. It is interesting to note that this ratio, when expressed in terms of weight (milligrams of Cd or Zn per grams of shoot), was larger than 1 for Ganges.
Influence of Fe Deficiency on Concentration-Dependent Kinetics of 109Cd and 65Zn Influx
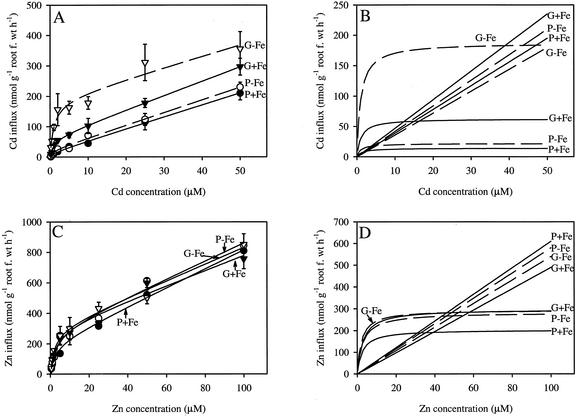
The concentration-dependent kinetics of 109Cd and 65Zn influx showed a saturable (hyperbolic) component and a linear component for both ecotypes (Fig. 2, A and C). To mathematically resolve these curves, we applied a Michaelis-Menten model combined with a linear component using SigmaPlot 5.0 (SPSS, Inc., Chicago). The linear and saturable components are shown separately in Figure 2, B and D, for Cd and Zn, respectively. This procedure to fit the curves was used by Lasat et al. (1996) to resolve the kinetic of influx of Zn in T. caerulescens. The linear component was believed to represent the apoplastically bound fraction that was not removed by the desorption procedure (Lasat et al., 1996). In all cases, the model fitted closely the experimental data as demonstrated by R2 values of between 0.96 and 0.99 (Table I).
Figure 2.
Concentration-dependent kinetics of Cd (A and B) and Zn (C and D) uptake in roots of Ganges (G) and Prayon (P) ecotypes of T. caerulescens grown in full nutrient solution (+Fe) or without Fe (−Fe) for 6 d. Data points represent mean values ± se, n = 3. The lines in A and C represent the best fit of the data using a Michaelis-Menten plus linear model. B and D show the dissected Michaelis-Menten and linear components separately.
Table I.
Parameters of the linear component and Michaelis-Menten model used to resolve the kinetic of influx curves in Figure 2, A and C
| Ecotype | Vmax | Km | Angular Coefficient | r2 |
|---|---|---|---|---|
| nmol g root fresh wt−1 h−1 | μm | nmol g root fresh wt−1 h−1 μm−1 | ||
| Cd | ||||
| Ganges + Fe | 62.6 (5.97) | 1.05 (0.30) | 4.7 (0.16) | 0.99 |
| Ganges − Fe | 187.6 (33.2) | 1.00 (0.53) | 3.6 (0.92) | 0.96 |
| Prayon + Fe | 14.0 (5.78) | 1.21 (1.43) | 3.9 (0.15) | 0.99 |
| Prayon − Fe | 21.8 (8.3) | 0.93 (1.09) | 4.2 (0.23) | 0.99 |
| Zn | ||||
| Ganges + Fe | 298.3 (66.0) | 2.83 (1.72) | 4.9 (0.86) | 0.97 |
| Ganges − Fe | 294.7 (39.4) | 2.10 (0.84) | 5.4 (0.54) | 0.99 |
| Prayon + Fe | 203.8 (33.1) | 2.53 (1.17) | 6.1 (0.44) | 0.99 |
| Prayon − Fe | 282.7 (56.9) | 2.80 (1.56) | 5.8 (0.74) | 0.98 |
Eight different concentrations of Cd (0.2 to 50 μm) or Zn (0.5 to 100 μm) were used to study the influx kinetics of Cd and Zn over a 20-min uptake period. ses are shown in parentheses.
Plants grown for 6 d in hydroponics without Fe showed initial symptoms of chlorosis, indicating Fe deficiency; however, no decrease in the biomass of roots or shoots were observed. The Cd influx was larger in Ganges than in Prayon both in Fe-sufficient and -deficient conditions (Fig. 2A). Also, in the Ganges ecotype, the rate of Cd influx was enhanced considerably by Fe deficiency, whereas in Prayon the response was small. A 3-fold increase in the maximal Cd influx (Vmax) was observed for Ganges when the plants were Fe deficient compared with the treatment where Fe was supplied (Table I). In the case of Prayon, the increase in Vmax in response to Fe deficiency was much less marked and statistically not significant. The Vmax for Cd was over 4 times larger in Ganges than in Prayon when the plants were grown in the presence of Fe. This difference increased to almost 9 times under the conditions of Fe deficiency. The saturable component of the Cd influx was characterized by similar Km values and no significant differences were observed between the ecotypes or as a result of the Fe status of the plants. Similarly, the angular coefficients characterizing the linear component of the kinetic of influx curves did not significantly vary between ecotypes and Fe treatments.
Figure 2C shows the concentration-dependent kinetic of 65Zn influx. No differences were observed between the two ecotypes and between the ±Fe treatments. There were no significant differences between the ecotypes and the Fe treatments in Vmax, Km, or the angular coefficients of the linear component (Fig. 2D; Table I).
Influence of Fe Deficiency on Simultaneous Uptake of 109Cd and 65Zn
The influence of Fe deficiency on the competition between Cd and Zn for uptake by the two ecotypes of T. caerulescens was investigated in this experiment. In both ecotypes, the uptake of Zn was not significantly influenced by the Fe status of the plants (Table II). In contrast, Cd uptake by Ganges was enhanced significantly (P < 0.05) by the −Fe treatment for 6 d. The effect of −Fe treatment on Cd uptake by Prayon was not significant. The Cd/Zn uptake ratio was always larger in Ganges than in Prayon, independent of the Fe status of the plants. Whereas Fe deficiency did not cause a significant change in this ratio in the Prayon ecotype, it significantly increased (P < 0.05) the Cd/Zn uptake ratio from 1.24 to 1.52 in Ganges.
Table II.
Simultaneous uptake of Cd and Zn and uptake of Fe in the two ecotypes of T. caerulescens and their response to Fe deficiency
| Ecotype | Cd | Zn | Cd/Zn | Fe |
|---|---|---|---|---|
| nmol g root fresh wt−1 h−1 | nmol g root fresh wt−1 h−1 | |||
| Ganges + Fe | 115.1 (8.1) | 92.4 (11.6) | 1.24 | 1,525 (237) |
| Ganges − Fe | 145.0 (7.5) | 95.4 (7.2) | 1.52 | 2,047 (99) |
| Prayon + Fe | 51.6 (4.1) | 82.3 (6.1) | 0.62 | 1,170 (195) |
| Prayon − Fe | 65.8 (5.3) | 95.9 (14.9) | 0.68 | 1,373 (95) |
Iron deficiency was induced for 6 d. For the Cd and Zn uptake experiment, the plants were transferred to uptake solutions containing 10 μm of radiolabelled CdCl2 and ZnCl2. The short-term uptake of 59Fe was determined by transferring the plants to an uptake solution containing 50 μm 59Fe-EDDHA. In both experiments, the uptake period was 20 min. ses are reported in parentheses; n = 4 for Cd and Zn, n = 5 for Fe.
The difference in Cd uptake between the two ecotypes (Table II) was not as large as that for the Vmax (Table I). This is likely because of the incomplete removal of apoplastically bound 109Cd by the desorption procedure, which would lead to a proportionally larger overestimation of 109Cd uptake by Prayon than by Ganges.
Short- and Long-Term Uptake of 59Fe by the Two Ecotypes
The 20-min uptake period showed no significant differences in terms of unidirectional influx rate of 59Fe in the roots of the two ecotypes. However, when Fe was supplied in the nutrient solution, slightly more Fe was taken up by Ganges than by Prayon (Table II). Fe deficiency induced an increment in the Fe uptake rate in both ecotypes. This increment was larger and statistically significant (P < 0.05) only in the Ganges ecotype.
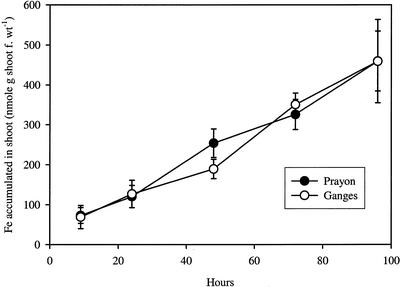
In the long-term experiment, the seedlings of T. caerulescens were grown in full hydroponic solution containing 59Fe-EDDHA for different time periods up to 4 d. After the uptake period, the 59Fe activity was measured in the shoots. This experimental design provides a combined measurement of both Fe uptake and root to shoot translocation. The results indicated that very similar amounts of Fe were accumulated in the shoots of the two populations (Fig. 3).
Figure 3.
Long-term accumulation of Fe in shoots of Ganges (G) and Prayon (P) ecotypes of T. caerulescens. Seedlings of both ecotypes were transferred to vessels containing a full nutrient solution with 59Fe(III)-EDDHA (3.7 KBq per pot). After 6, 24, 48, 72, and 96 h, plants were harvested and 59Fe in the shoots determined. Data points represent mean values ± se, n = 5.
Gene Cloning
Two genes belonging to the ZIP family were investigated because they have been previously implicated in Cd uptake. ZNT1 was originally cloned from the Prayon ecotype of T. caerulescens (Pence et al., 2000), and is responsible for Zn uptake in T. caerulescens but can also mediate low-affinity Cd transport (Pence et al., 2000). IRT1 could account for the enhanced Cd uptake seen in Fe-deficient pea seedlings (Cohen et al., 1998). Partial cDNA clones of an Fe transporter (TcIRT1-G; GenBank accession no. AJ320253) and a Zn transporter (TcZNT1-G; GenBank accession no. AJ313521) were isolated from Ganges root tissue by reverse transcriptase (RT)-PCR. The cloned TcZNT1-G fragment is 1,078 bp long, spanning nucleotides 36 through 1,113 of Prayon ZNT1 (GenBank accession no. AF133267; Pence et al., 2000). Over this region, TcZNT1-G from Ganges shares 99.5% nucleotide identity with the ZNT1 gene from Prayon, with 99.4% identity and 99.7% similarity at the amino acid level.
The TcIRT1-G cDNA fragment has 87.2% nucleotide identity to the Fe transporter IRT1 characterized from Arabidopsis (GenBank accession no. U27590; Eide et al., 1996) with 89.3% identity and 91.8% similarity to its amino acid sequence. Because this is the first report of an IRT gene from T. caerulescens, the full-length cDNA sequence was cloned using 3′- and 5′-RACE techniques. The full-length TcIRT1-G cDNA clone is 1,360 bp long to the poly(A+) tail and contains an open reading frame encoding a 346-amino acid polypeptide. The open reading frame of TcIRT1-G is flanked by 48 bp of untranslated sequence at the 5′ end and by 274 bp of untranslated sequence, excluding the poly(A+) tail, at the 3′ end. Over the open reading frame, TcIRT1-G has 88.2% nucleotide identity to Arabidopsis IRT1, with 90.2% identity and 92.9% similarity at the amino acid level. TcIRT1-G also has 74.6% nucleotide identity to Arabidopsis IRT2 (GenBank accession no. T04324; Vert et al., 2001) with 70.6% identity and 77.8% similarity at the amino acid level. As with the Arabidopsis IRT genes, the deduced TcIRT1-G polypeptide is predicted to consist of eight membrane-spanning regions, with a putative heavy metal binding site between transmembrane domains III and IV (Eng et al., 1998). Both TcZNT1-G and TcIRT1-G cDNAs are predicted to be members of the ZIP family of metal ion transporters (Guerinot, 2000).
Expression Analysis
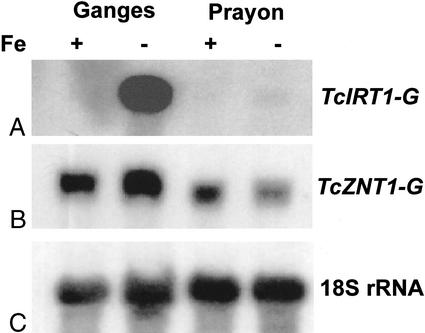
The cloned TcZNT1-G and TcIRT1-G RT-PCR products were used as probes in northern blotting (Fig. 4). Expression of the respective genes in roots of T. caerulescens Ganges and Prayon ecotypes was studied under Fe-sufficient and -deficient conditions. No change in the abundance of TcZNT1-G mRNA was observed between the Fe treatments or between the two ecotypes. In contrast, abundance of the TcIRT1-G mRNA was shown to be greatly increased in Ganges root tissue under Fe-deficient conditions. Prolonged exposure of this northern enabled a small increase in message abundance to be observed in the roots of Prayon.
Figure 4.
Fe and Zn transporter expression in T. caerulescens ecotypes. mRNA was detected by northern-blot analysis of total RNA extracted from roots of Ganges and Prayon. The lanes contained 10 μg of total RNA each. A, Expression of TcIRT1-G in the roots of Ganges and Prayon. B, Expression of TcZNT1-G in the roots of Ganges and Prayon. C, Hybridization of the blot with 18S rRNA used as loading control.
DISCUSSION
In previous papers, we have reported and physiologically characterized the differences between the Ganges and Prayon ecotypes of T. caerulescens in terms of Cd uptake (Lombi et al., 2000, 2001). The present paper investigates the response of these two ecotypes to Fe deficiency and its influence in terms of Cd and Zn uptake to provide new indications regarding the Cd uptake mechanism in T. caerulescens.
The long-term experiment conducted using different concentrations of Fe-EDDHA in solution showed that Cd and Zn accumulation patterns were different between the two ecotypes. Cd concentrations were larger in Ganges than in Prayon. When Fe was normally supplied in nutrient solution, Cd concentration in Ganges was approximately 4 times larger than in Prayon. This result is in agreement with a previous finding that showed approximately 3 times more Cd in Ganges than in Prayon when grown in soil amended to different Cd concentrations (Lombi et al., 2000). When the concentration of Fe-EDDHA in solution was reduced the differences between the ecotypes increased, with over 7 times higher Cd concentration in the shoots of Ganges than that of Prayon. It is clear that Fe deficiency greatly enhanced Cd uptake in the Ganges ecotype, but had little effect in the Prayon ecotype. Other authors have also reported an increase in metal accumulation as a consequence of Fe deficiency; for instance, Rodecap et al. (1994) showed that Fe deficiency induced a higher accumulation of Cd in Arabidopsis plants. In our experiment, Zn accumulation was less influenced by the Fe status of the plants than Cd.
The results of the long-term accumulation experiment reported above are in agreement with short-term uptake studies of the kinetics of Cd and Zn influx performed using radiotracers. In both Cd and Zn uptake kinetic studies, the curves were characterized by a saturable and a linear component in both ecotypes (Fig. 2). The saturable component is generally considered as true transport across the plasma membrane (Lasat et al., 1996; Cohen et al., 1998). The Vmax of Cd influx of Ganges increased markedly in response to Fe deficiency (Table I). In contrast, the response in Vmax was not significant in Prayon. As a result, the difference between the two ecotypes in the Vmax for Cd influx increased from 4-fold under Fe-sufficient conditions to 9-fold under Fe-deficient conditions. Cohen et al. (1998) previously showed that Fe deficiency increased the Vmax for Cd influx in the nonaccumulator pea seedlings by nearly 7-fold. In contrast to Cd influx, the kinetics of Zn uptake did not significantly change in either ecotype when Fe deficiency was induced (Table I). The results of the kinetic studies are confirmed by the experiment in which the simultaneous uptake of 109Cd and 65Zn and its response to Fe deficiency was investigated. In this case, Fe deficiency again significantly enhanced Cd uptake by the Ganges ecotype but had little effect on Cd uptake by Prayon or on Zn uptake by either ecotype (Table II). As a result, the ratio between Cd and Zn uptake increased in Ganges but not in Prayon.
The molecular study provides a possible explanation to the physiological data presented. The abundance of TcZNT1-G mRNA (Fig. 4) is independent of Fe status and similar between ecotypes. This corresponds with the Zn uptake observed in the physiological studies. Expression of TcZNT1-G was observed in both ecotypes, despite the fact that the plants were grown with sufficient levels of Zn (5 μm). This confirms the previous studies by Pence et al. (2000) and Assunção et al. (2001). The lack of difference between the two ecotypes and between the Fe-sufficient and -deficient plants indicates that TcZNT1-G is not responsible for the superior ability of the Ganges ecotype to take up Cd.
The greatly increased transcript abundance of TcIRT1-G in the Ganges ecotype in response to Fe deficiency (Fig. 4) corresponds to the greatly increased Vmax for Cd influx (Table I) and Cd accumulation in the shoots (Fig. 1). The much smaller induction of the expression of TcIRT1-G is also consistent with the small increase in the Vmax for Cd influx in the Prayon ecotype. The results suggest that overexpression of TcIRT1-G may be responsible for enhanced Cd uptake in Ganges in response to Fe deficiency. The ability of IRT1 to transport Cd has been established. Cohen et al. (1998) also suggested that increased expression of IRT1 may be linked to the increased Cd uptake by pea under Fe deficiency. Although TcIRT1-G may be involved in the enhanced Cd uptake in the Ganges ecotype under Fe deficiency, it is not clear whether it is linked to the large ecotypic difference in Cd uptake under Fe sufficiency.
Apart from IRT1, the Nramp family of Fe transporters has been shown to be capable of transporting Cd (Thomine et al., 2000). Whether Nramps are involved in Cd uptake in the hyperaccumulator T. caerulescerns was not investigated in this study.
Our results of the 59Fe uptake experiments indicate that under Fe-sufficient conditions, the uptake and root to shoot translocation of Fe were not significantly different between the two ecotypes (Table II; Fig. 3). On the other hand, Fe deficiency enhanced short-term uptake of Fe that is statistically significant only in the Ganges ecotype. Again, the enhanced uptake of Fe in Fe-deficient Ganges plants seems to be linked to the overexpression of Fe transporter gene TcIRT1-G (Fig. 4). The low expression of IRT1 in the Prayon ecotype, even under Fe deficiency, implies that this ecotype may rely on other Fe transporters (e.g. other ZIP and Nramp transporters) to acquire Fe.
AtIRT1 is also capable of complementing Zn uptake in a yeast (Saccharomyces cerevisiae) mutant defective for Zn uptake (Korshunova et al., 1999). Therefore, it is not clear why Zn uptake was not enhanced under Fe-deficient conditions. However, Rogers et al. (2000) demonstrated by heterologous expression in yeast that the alteration of single amino acids in AtIRT1 could alter metal selectivity. For instance, replacement of a Glu residue at position 103 with Ala eliminated the ability of AtIRT1 to transport Zn. The work of Rogers et al. (2000) clearly showed how the selectivity of metal transport in proteins belonging to the ZIP family might be related to specific residues.
At the moment, our knowledge about the mechanism of Cd uptake in Thlaspi spp. is far from complete. For instance, only very recently Assunção et al. (2001) have cloned, in different accessions of T. caerulescens, another two genes, ZTP1 (an AtZAT homolog) and ZNT2 (homolog of ZNT1), that encode for Zn transporters. Furthermore, Persans et al. (2001) have characterized genes from Thlaspi goesingense encoding putative vacuolar metal ion transport proteins (TgMTPs) probably responsible for Ni accumulation within leaf vacuoles. This indicates that the molecular mechanism responsible for metal uptake in hyperaccumuator plants is probably very complex. The present paper provides indications that, under Fe-deficient conditions, Cd uptake in the Ganges ecotype may be enhanced by up-regulation of the Fe transporter TcIRT1-G. Further studies are ongoing to functionally characterize this gene and to further investigate its regulation in T. caerulescens under Fe-sufficient and -deficient conditions.
MATERIALS AND METHODS
Plant Materials and Culture
Seeds of the two ecotypes of Thlaspi caerulescens J & C Presl were collected in Belgium (Prayon ecotype) and near St. Laurent le Minier, Southern France (Ganges ecotype). The French site is described in more detail by Robinson et al. (1998). The seeds were germinated on a mixture of perlite and vermiculite moistened initially with deionized water and, after germination, with nutrient solution. After 20 to 30 d, seedlings were transferred to vessels (three seedlings per vessel) filled with a nutrient solution containing: 1,000 μm Ca(NO3)2, 500 μm MgSO4, 50 μm K2HPO4, 100 μm KCl, 10 μm H3BO3, 1.8 μm MnSO4, 0.2 μm Na2MoO4, 0.31 μm CuSO4, 0.5 μm NiSO4, 50 μm Fe(III)-EDDHA, and 5 μm ZnSO4 (Shen et al., 1997). Solution pH was buffered to approximately 6.0 with 2,000 μm MES (pH adjusted with KOH). The plants were grown and the experiments conducted in a controlled environment with the following conditions: 16-h day length with a light intensity of 350 μmol photons m−2 s−1 supplied by fluorescent tubes, 20°C/16°C day/night temperature, and 60% to 70% relative humidity. The hydroponic solutions used were continuously aerated and renewed every 3 d.
Cd and Zn Accumulation and Growth Response to Fe Status
Eight days after plants were transferred to the nutrient solution described above (three seedlings per 250-mL vessel), different concentrations of Fe were imposed. Fe was supplied as Fe(III)-EDDHA at the concentrations of 0, 5, 15, 30, and 60 μm. Each treatment was replicated four times. All treatments contained 5 μm CdCl2 and 5 μm ZnSO4. Nutrient solutions were renewed on d 5. The plants were harvested on d 10. Roots and shoots were rinsed thoroughly with deionized water and dried at 60°C for 48 h. Subsamples (0.5 g) of finely ground tissue were digested with concentrated HNO3 and the concentrations of Cd and Zn in the digest were determined using inductively coupled plasma-atomic emission spectrometry (Spectro Analytical Instruments, Kleve, Germany).
Influence of Fe Deficiency on Concentration-Dependent Kinetics of 109Cd and 65Zn Influx
Seedlings were grown in hydroponic vessels (three seedlings in each 50-mL vessel) with full nutrient solution for 10 d. After this period, Fe deficiency was induced in one-half of the plants replacing the full nutrient solution with a nutrient solution without Fe for 6 d. The procedure described by Lasat et al. (1996) for the determination of Zn influx kinetics was followed. Eight different concentrations of Cd (0.2–50 μm) or Zn (0.5–100 μm) were used to study the influx kinetics of Cd and Zn, separately. The isotopes 109Cd or 65Zn were added to the uptake solutions to give 3.7 KBq per vessel. Each concentration was replicated three times. After 20 min of uptake, the seedlings were quickly rinsed with the unlabeled pretreatment solution, and then transferred to vessels containing ice-cold desorption solutions (2,000 μm K-MES, 5,000 μm CaCl2, and 100 μm CdCl2, or 100 μm ZnCl2 for the Cd and Zn uptake series, respectively). Lasat et al. (1996) showed that this desorption step was effective in removing most of the Zn adsorbed on cell walls of T. caerulescens and Thlaspi arvense roots. After desorption, the seedlings were separated into roots and shoots, blotted dry, and weighed. Roots and shoots were transferred into radioactivity counting vials, to which 4 mL of 5 m HNO3 was added. After 3 d, 109Cd and 65Zn were analyzed by gamma spectroscopy (Canberra Packard Auto Gamma 5780, Meriden, CT).
Influence of Fe Deficiency on Simultaneous Uptake of 109Cd and 65Zn
Thirty-day-old seedlings were transferred into vessels containing a full nutrient solution and grown for 10 d. Fe deficiency was then induced in one-half of the plants as described above. After 6 d, the plants were transferred into the pretreatment solution overnight (Lasat et al., 1996). Then, the plants were transferred to uptake solutions containing 10 μm CdCl2 and 10 μm ZnCl2 spiked with carrier-free 109Cd and 65Zn to give 3.7 KBq of each isotope per vessel. After a 20-min uptake period, the seedlings were removed from the uptake solution and treated as described above to desorb the apoplastically bound 109Cd and 65Zn. Roots were then transferred to counting vials and, after partial digestion using 4 mL of M HNO3, 109Cd and 65Zn were analyzed by gamma spectroscopy.
Long- and Short-Term Uptake of 59Fe by the Two Ecotypes
These experiments were designed to assess whether the two ecotypes take up Fe from the nutrient solution at the same rate. Seedlings of the two ecotypes were grown in hydroponic vessels (three seedlings in each 50-mL vessel) with full nutrient solution for 10 d.
For the short-term experiment, Fe deficiency was induced in one-half of the plants over a 6-d period as described above. After this period, Fe-deficient and -sufficient seedlings were transferred into a pretreatment solution overnight (Lasat et al., 1996). The short-term uptake of 59Fe was performed exposing the seedlings to an uptake solution containing 50 μm 59Fe-EDDHA (37 KBq per vessel). Five replicates per treatment were used. After a 20-min uptake period, the seedlings were transferred to a rinsing solution of 5 mm MgSO4. Then, the adsorbed 59Fe was desorbed for 10 min using the procedure described by Grusak et al. (1990). After desorption, the seedlings were separated in roots and shoots, blotted dry, and weighed.
For the long-term uptake experiment, the seedlings were transferred to vessels containing a full nutrient solution with 59Fe(III)-EDDHA (3.7 KBq per pot). After 6, 24, 48, 72, and 96 h, plants in five vessels per ecotype were harvested to determine 59Fe uptake from the roots and translocated to the shoot. At the end of the uptake period, the seedlings were rinsed and separated into roots and shoots, blotted dry, and weighed. Radioactivity of 59Fe was determined using gamma spectroscopy.
Gene Cloning
The T. caerulescens gene TcZNT1-G was cloned by RT-PCR from the Ganges ecotype total RNA using oligonucleotide primer sequences obtained from the original accession (GenBank accession no. AF133267; Pence et al., 2000). The T. caerulescens IRT1 (TcIRT1-G) gene was cloned using degenerate oligonucleotide primers designed from conserved regions of the aligned sequence data of all known plant IRTs deposited in the GenBank database. RT-PCR amplification was performed as described by Frohman et al. (1995). The primers used were: TcZNT1-G primer 1, 5′-GTTCAGATCAGGAAGAGATC-3′; TcZNT1-G primer 2, 5′-ATCAGCTGCGATTAGCTCCAC-3′; TcIRT1-G primer 1, 5′-GCKYTRYCYYTHAAARTCATAGC-3′; and TcIRT1-G primer 2, 5′-AGCRGAKSATSCATTDAGHARTCCAAC-3′. Products from RT-PCR reactions were cloned into the pGEM-T Easy vector (Promega, Madison, WI) for transformation into Escherichia coli. Sequencing of the plasmid insert was performed on an ABI Prism 310 genetic analyser using Big Dye sequencing reagent (PE-Applied Biosystems, Foster City, CA). For TcIRT1-G, a full-length clone was obtained using 3′- and 5′-RACE techniques as per the manufacturer's instructions (3′-/5′-RACE kit, Roche Diagnostics, Mannheim, Germany) and sequenced as described above.
Expression Analysis
Seedlings of both ecotypes were grown in hydroponic vessels with full nutrient solution for 10 d. Fe deficiency was successively induced in one-half of the plants replacing the full nutrient solution with a nutrient solution without Fe for 6 d. RNA was extracted from Prayon and Ganges tissues by the method of Verwoerd et al. (1989). Ten micrograms of total RNA from roots of Fe-sufficient and -deficient plants was separated on a 1% (w/v) agarose gel containing 3% (v/v) formaldehyde and blotted onto a nylon membrane as described in Sambrook et al. (1989). Membranes were probed overnight at 65°C using TcZNT1-G and TcIRT1-G gene fragments radiolabeled with [α32P]dCTP using Prime-A-Gene oligonucleotide labeling system (Promega). Equal loading of RNA in each lane was verified by probing with a constitutively expressed 18S rDNA fragment. Membranes were washed to high stringency in 1× SSC and 0.1% (w/v) SDS at 65°C before visualizing on Biomax MS autoradiography film (Eastman-Kodak, Rochester, NY). Between hybridizations, probes were stripped from nylon membranes with boiling 0.1% (w/v) SDS.
ACKNOWLEDGMENTS
We thank Drs. Mike McLaughlin, Rebecca Hamon, and David Evans for the valuable discussions and suggestions.
Footnotes
This work was supported by the Directorate General XII of the European Commission, by Rio Tinto Technology, and by the Biotechnology and Biological Sciences Research Council of the UK (grant to IACR-Rothamsted).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.010731.
LITERATURE CITED
- Assunção AGL, De Costa Martins P, De Folter S, Vooijs R, Schat H, Aarts MGM. Elevated expression of metal transporter genes in three accessions of the metal hyperaccumulator Thlaspi caerulescens. Plant Cell Environ. 2001;24:217–226. [Google Scholar]
- Baker AJM, McGrath SP, Reeves RD, Smith JAC. Metal hyperaccumulator plants: a review of the ecology and physiology of a biological resource for phytoremediation of metal-polluted soils. In: Terry N, Banuelos G, Vangronsveld J, editors. Phytoremediation of Contaminated Soil and Water. Boca Raton, FL: Lewis Publisher; 2000. pp. 85–107. [Google Scholar]
- Baker AJM, Reeves RD, Hajar ASM. Heavy metal accumulation and tolerance in British populations of the metallophyte Thlaspi caerulescens J. & C. Presl (Brassicaceae) New Phytol. 1994;127:61–68. doi: 10.1111/j.1469-8137.1994.tb04259.x. [DOI] [PubMed] [Google Scholar]
- Clemens S, Antosiewicz DM, Ward JM, Schachtman DP, Schroeder JI. The plant cDNA LCT1 mediates the uptake of calcium and cadmium in yeast. Proc Natl Acad Sci USA. 1998;95:12043–12048. doi: 10.1073/pnas.95.20.12043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen CK, Fox TC, Garvin DF, Kochian LV. The role of iron-deficiency stress responses in stimulating heavy-metal transport in plants. Plant Physiol. 1998;116:163–1072. doi: 10.1104/pp.116.3.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eide D, Broderius M, Fett J, Guerinot ML. A novel iron-regulated metal transporter from plants identified by functional expression in yeast. Proc Nat Acad Sci USA. 1996;93:5624–5628. doi: 10.1073/pnas.93.11.5624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eng BH, Guerinot ML, Eide D, Saier MH. Sequence analyses and phylogenetic characterization of the ZIP family of metal ion transport proteins. J Membr Biol. 1998;166:1–7. doi: 10.1007/s002329900442. [DOI] [PubMed] [Google Scholar]
- Frohman MA. Rapid amplification of cDNA ends. In: Dieffenbach CW, Dveksler GS, editors. PCR Primer: A Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1995. pp. 381–410. [Google Scholar]
- Grusak MA, Welch RM, Kochian LV. Physiological characterization of a single-gene mutant Pisum sativum exhibiting excess iron accumulation. Plant Physiol. 1990;93:976–981. doi: 10.1104/pp.93.3.976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerinot ML. The ZIP family of metal transporters. Biochim Biophys Acta. 2000;1465:190–198. doi: 10.1016/s0005-2736(00)00138-3. [DOI] [PubMed] [Google Scholar]
- Korshunova YO, Eide D, Clark WG, Guerinot ML, Pakrasi HB. The IRT1 protein from Arabidopsis thaliana is a metal transporter with a broad substrate range. Plant Mol Biol. 1999;40:37–44. doi: 10.1023/a:1026438615520. [DOI] [PubMed] [Google Scholar]
- Lasat MM, Baker AJM, Kochian LV. Physiological characterization of root Zn2+ absorption and translocation to shoots in Zn hyperaccumulator and nonaccumulator species of Thlaspi. Plant Physiol. 1996;112:1715–1722. doi: 10.1104/pp.112.4.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombi E, Zhao FJ, Dunham SJ, McGrath SP. Cadmium accumulation in populations of Thlaspi caerulescens and Thlaspi goesingense. New Phytol. 2000;145:11–20. [Google Scholar]
- Lombi E, Zhao FJ, McGrath SP, Young S, Sacchi GA. Physiological evidence for a high affinity cadmium transporter in a Thlaspi caerulescens ecotype. New Phytol. 2001;149:53–60. doi: 10.1046/j.1469-8137.2001.00003.x. [DOI] [PubMed] [Google Scholar]
- Pence NS, Larsen PB, Ebbs SD, Letham DLD, Lasat MM, Garvin DF, Eide D, Kochian LV. The molecular physiology of heavy metal transporter in the Zn/Cd hyperaccumulator Thlaspi caerulescens. Proc Natl Acad Sci USA. 2000;97:4956–4960. doi: 10.1073/pnas.97.9.4956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persans MW, Nieman K, Salt DE. Functional activity and role of cation-efflux family members in Ni hyperaccumulation in Thlaspi goesingense. Proc Natl Acad Sci USA. 2001;98:9995–10000. doi: 10.1073/pnas.171039798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson BH, Leblanc A, Petit D, Brooks RR, Kirkman JH, Gregg PEH. The potential of Thlaspi caerulescens for phytoremediation of contaminated soils. Plant Soil. 1998;203:47–56. [Google Scholar]
- Rodecap KD, Tingey DT, Lee EH. Iron nutrition influence on cadmium accumulation by Arabidopsis thaliana (L) Heynh. J Environ Qual. 1994;23:239–246. [Google Scholar]
- Rogers EE, Eide DJ, Guerinot ML. Altered selectivity in an Arabidopsis metal transporter. Proc Natl Acad Sci USA. 2000;97:12356–12360. doi: 10.1073/pnas.210214197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- Shen ZG, Zhao FJ, McGrath SP. Uptake and transport of zinc in the hyperaccumulator Thlaspi caerulescens and the non-hyperaccumulator Thlaspi ochroleucum. Plant Cell Environ. 1997;20:898–906. [Google Scholar]
- Thomine S, Wang R, Ward JM, Crawford NM, Schroeder JI. Cadmium and iron transport by members of a plant metal transporter family in Arabidopsis with homology to Nramp genes. Proc Natl Acad Sci USA. 2000;97:4991–4996. doi: 10.1073/pnas.97.9.4991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vert G, Briat JF, Curie C. Arabidopsis IRT2 gene encodes a root-periphery iron transporter. Plant J. 2001;26:181–189. doi: 10.1046/j.1365-313x.2001.01018.x. [DOI] [PubMed] [Google Scholar]
- Verwoerd TC, Dekker BMM, Hoekema A. A small-scale procedure for the rapid isolation of plant RNAs. Nucleic Acids Res. 1989;17:2362. doi: 10.1093/nar/17.6.2362. [DOI] [PMC free article] [PubMed] [Google Scholar]