Abstract
Pyruvate,orthophosphate (Pi) dikinase (PPDK) is best recognized as a chloroplastic C4 cycle enzyme. As one of the key regulatory foci for controlling flux through this photosynthetic pathway, it is strictly and reversibly regulated by light. This light/dark modulation is mediated by reversible phosphorylation of a conserved threonine residue in the active-site domain by the PPDK regulatory protein (RP), a bifunctional protein kinase/phosphatase. PPDK is also present in C3 plants, although it has no known photosynthetic function. Nevertheless, in this report we show that C3 PPDK in leaves of several angiosperms and in isolated intact spinach (Spinacia oleracea) chloroplasts undergoes light-/dark-induced changes in phosphorylation state in a manner similar to C4 dikinase. In addition, the kinetics of this process closely resemble the reversible C4 process, with light-induced dephosphorylation occurring rapidly (≤15 min) and dark-induced phosphorylation occurring much more slowly (≥30–60 min). In intact spinach chloroplasts, light-induced dephosphorylation of C3 PPDK was shown to be dependent on exogenous Pi and photosystem II activity but independent of electron transfer from photosystem I. These in organello results implicate a role for stromal pools of Pi and adenylates in regulating the reversible phosphorylation of C3-PPDK. Last, we used an in vitro RP assay to directly demonstrate ADP-dependent PPDK phosphorylation in desalted leaf extracts of the C3 plants Vicia faba and rice (Oryza sativa). We conclude that an RP-like activity mediates the light/dark modulation of PPDK phosphorylation state in C3 leaves and chloroplasts and likely represents the ancestral isoform of this unusual and key C4 pathway regulatory “converter” enzyme.
Pyruvate,orthophosphate (Pi) dikinase (PPDK; EC 2.7.9.1) is a well-known enzyme of the C4 photosynthetic pathway where it catalyzes the ATP- and Pi-dependent formation of phosphoenolpyruvate (PEP), the primary CO2 acceptor molecule, from pyruvate:
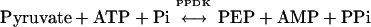 |
Consistent with its being a rate-limiting enzyme in the C4 cycle, PPDK activity is regulated in a reversible, light-dependent manner so the overall pathway can function optimally in net CO2 assimilation (Hatch, 1987; Furbank et al., 1997). This posttranslational regulation is conferred by reversible phosphorylation of a “target” Thr residue (Thr-456 in maize [Zea mays] C4 PPDK) proximal to a catalytically essential (phospho) His (His-458 in maize), with the enzyme being inactive in its threonyl-phosphorylated state. A single, bifunctional protein kinase/phosphatase, named the PPDK regulatory protein (RP), catalyzes this regulatory phosphorylation/dephosphorylation cycle (Fig. 1; Burnell and Hatch, 1984, 1985, 1986; Roeske and Chollet, 1987; Ashton et al., 1990). Along with its target enzyme, RP is specifically localized in the chloroplast stroma of the C4 mesophyll cell. It is a highly unusual and unique RP in at least three important respects. First, it is bifunctional in that it catalyzes both PPDK inactivation (phosphorylation) and activation (dephosphorylation). This is quite rare because most regulatory phosphorylation cycles have separate kinase and phosphatase enzymes (Hanks and Hunter, 1995; Smith and Walker, 1996; Hardie, 1999). Second, it uses ADP instead of ATP as the phosphoryl donor. Third, it employs a Pi-dependent, inorganic pyrophosphate (PPi)-forming phosphorolytic dephosphorylation mechanism, as opposed to simple hydrolysis as in most protein phosphatases. As is the case with other C4 pathway enzymes, such as PEP carboxylase (PEPc) and NADPH-malate dehydrogenase, PPDK is also present in C3 plants, and, likewise, this isoform is not thought to participate directly in photosynthesis. The dikinase found in C3 plants is highly homologous to the C4 enzyme with respect to structure and biochemical properties (Aoyagi and Bassham, 1984; Hata and Matsuoka, 1987; Burnell, 1990; Rosche et al., 1994; Fisslthaler et al., 1995; Imaizumi et al., 1997). It is interesting that the specific target Thr residue for RP in C4 PPDK is conserved in all C3 angiosperm (and prokaryotic) dikinase genes examined to date (Rosche et al., 1994; Fisslthaler et al., 1995; Agarie et al., 1997; Imaizumi et al., 1997; Wei et al., 2000). In C3 plants, PPDK is a ubiquitous but very low-abundance enzyme found in the cytosol (Aoyagi and Bassham, 1985; Aoyagi and Chua, 1988; Moons et al., 1998; Nomura, et al., 2000) and chloroplasts (Aoyagi and Bassham, 1985; Hocking and Anderson, 1986). Non-photosynthetic PPDK is apparently more abundant in the endosperm of developing cereal seeds, where it is expressed in a developmentally regulated manner (Gallusci et al., 1996; Nomura, et al., 2000). Although PPDK in C3 plants occurs with ubiquity, its exact metabolic functions are unknown but are likely to be multifaceted because of its different cellular and subcellular locations. Whatever the function(s) of PPDK is in C3 plants, its conversion from a non-photosynthetic role to a photosynthetic one in mesophyll chloroplasts of C4 leaves was a transition repeated independently in a diverse range of angiosperm families during the evolution of C4 photosynthesis. This implies a more or less common evolutionary pathway for C4 photosynthesis facilitated by the preexistence of homologs of the C4 cycle enzymes in C3 plants (Edwards et al., 2001). Potentially more problematic for understanding how the C4 pathway emerged independently are the origins of certain regulatory “converter” enzymes that fulfill the crucial role of controlling carbon flux through the C4 pathway. Two examples are the PEPc regulatory enzymes, PEPc kinase (PpcK) and PEPc phosphatase (Vidal and Chollet, 1997). Both of these “converter” enzymes can be found across the spectrum of C4 species and so must be assumed not to have evolved de novo each time PEPc acquired its photosynthetic role. This has now been inferred by several reports that have characterized the reversible phosphorylation of PEPc in various tissues of C3 plants, especially during its role in C/N metabolism (Duff and Chollet, 1995; Zhang et al., 1995; Li et al., 1996). Further, C3 leaf PpcK has been linked to a complex signaling pathway that includes a light/dark regulatory component (Li et al., 1996), which is also the primary regulatory function this Ser/Thr kinase fulfills in controlling C4 PEPc (Vidal and Chollet, 1997). In this report, we provide evidence that the C3 precursor to another C4 regulatory “converter” enzyme, PPDK-RP, or an RP-like activity, functions in chloroplasts of C3 leaves. We also demonstrate that the opposing C3 PPDK phosphorylation and dephosphorylation activities are regulated in planta and in organello in a manner similar to C4 RP.
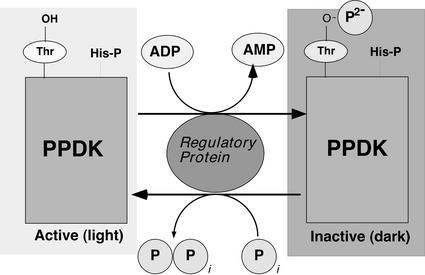
Figure 1.
Simplified model of the regulatory phosphorylation of C4 PPDK by its bifunctional RP in the mesophyll-chloroplast stroma. As a consequence of dark-induced increases in stromal [ADP], the RP-catalyzed kinase reaction is favored and the “target” enzyme is inactivated by introduction of a dianionic (2−) charge into the active-site domain. In the light, stromal ADP levels decrease, favoring the RP-catalyzed dephosphorylation reaction and subsequent restoration of PPDK activity. The strictly conserved, catalytically essential His-P residue at the P+2 position (e.g. His-458 in maize C4 PPDK; Chastain et al., 2000; Wei et al., 2000) is also indicated. Modified from Figure 50 in Malkin and Niyogi (2000).
RESULTS
Reversible Phosphorylation of PPDK in C3 Leaves
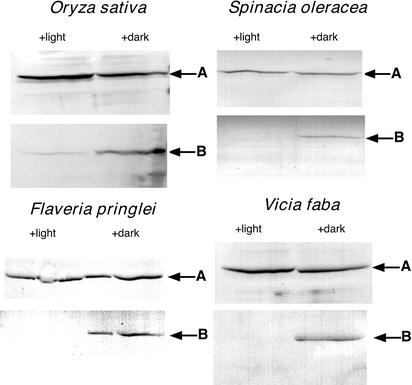
Our principal experimental approach for assessing the in vivo activity of a putative C3 RP was to employ an antibody that specifically recognizes the phosphorylated regulatory site (Thr-456 in maize). The antigen used to generate this antibody was a synthetic phosphopeptide conjugate corresponding to the strictly conserved phosphorylation domain of plant PPDK (maize C4 PPDK residues 445–464; Chastain et al., 2000). This antibody has proven invaluable for assessing the light-/dark-induced reversible phosphorylation of C4 PPDK via immunoblot analysis (Chastain et al., 2000). The use of this phosphorylation state-specific antibody was complemented with a polyclonal antibody raised against the maize leaf dikinase monomer (Budde and Chollet, 1986). This latter antibody, which detects both the phospho and dephospho forms of PPDK, allowed for the normalization of total PPDK on immunoblots. Initial attempts to detect phospho-PPDK on immunoblots using crude C3 leaf extracts proved ineffective, presumably because of the very low abundance this protein. For example, we could detect only a very faint signal on blots probed with the standard PPDK antibody and no signal on blots probed with phospho-PPDK antibody, even with unusually high loads of soluble protein (approximately 200 μg) per lane (data not shown). To visualize phospho-PPDK on immunoblots, we found it necessary to first concentrate C3 leaf PPDK from crude soluble extracts by ammonium sulfate fractionation. This facile step effectively concentrated PPDK for routine detection on immunoblots using both phospho-PPDK and standard PPDK antibodies and provided the basis for ascertaining light-/dark-induced changes in phosphorylation state of PPDK in C3 leaves. Of the many C3 dicots and monocots we have examined, including the four species illustrated in Figure 2 (rice [Oryza sativa], spinach [Spinacia oleracea], Flaveria pringlei, and Vicia faba), all showed PPDK phosphorylation when leaves were dark adapted for 3 h or dephosphorylation when subsequently illuminated for 1 h. The ability of the affinity-purified maize phosphopeptide antibody to reliably detect a diverse range of C3- and C4-phospho PPDKs with little or no background hybridization (see Fig. 2) may readily be explained by the highly conserved sequence of the PPDK active-site regulatory domain in angiosperms and microorganisms (Wei et al., 2000).
Figure 2.
PPDK in C3 leaves undergoes light-/dark-induced reversible phosphorylation. Displayed are duplicate immunoblots of C3 leaf soluble proteins concentrated by 35% to 55% saturation ammonium sulfate fractionation. A, Blots probed with standard PPDK antibody; B, blots probed with phospho-PPDK antibody. Arrows indicate the band corresponding to the ≈95-kD PPDK monomer as estimated by molecular mass standards on the same blot. Leaves were dark adapted for 3 h (+ dark lanes) and then illuminated for 1 h at approximately 800 μmol m−2 s−1 (+ light lanes) before extraction. Each lane was loaded with 100 μg of protein. All experiments were repeated independently at least three times.
Kinetic Analysis of the Dark-/Light-Induced Reversible Phosphorylation of PPDK in C3 and C4 Leaves
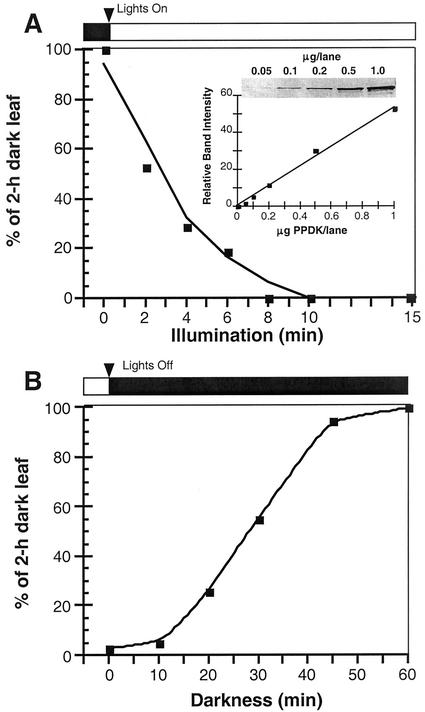
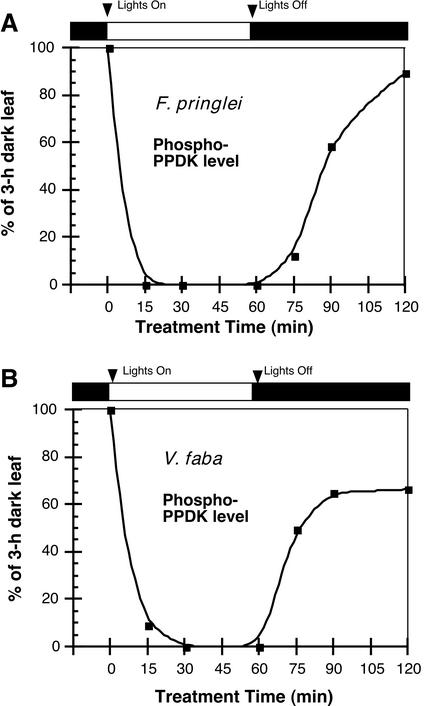
In C4 plants, PPDK light activation (dephosphorylation) and dark inactivation (phosphorylation) as catalyzed by RP (see Fig. 1) have been previously examined using indirect measurements of extractable leaf or mesophyll protoplast and chloroplast dikinase activity. In this manner, the “target” enzyme's phosphorylation state is estimated by its level of activity. Using this indirect method, time course experiments measuring C4 PPDK activation and deactivation during light/dark transitions have been published (Yamamoto et al., 1974; Nakamoto and Edwards, 1986; Roeske and Chollet, 1989; Nakamoto and Young, 1990). We performed similar kinetic studies with representative C4 (maize) and C3 (V. faba and F. pringlei) leaves, but directly assessed PPDK phosphorylation state during light/dark transitions. This was accomplished by image analysis of phospho-PPDK bands representing each time point on immunoblots (Figs. 3 and 4). For maize, such analysis showed that when leaves were illuminated after a 2-h period in the dark, the rate of PPDK dephosphorylation was very rapid and nearly complete after 8 min (Fig. 3A). When these illuminated (1 h) leaves were returned to darkness, the rate of PPDK phosphorylation was much slower, requiring approximately 1 h for maximum modification (Fig. 3B). These data agree well with the aforementioned studies that measured changes in extractable dikinase activity from intact C4 leaves and isolated mesophyll protoplasts and chloroplasts. The analysis of this model C4 plant documented the validity of our immunoblot assay and provided the basis for comparing the kinetics of light-/dark-induced, reversible changes in PPDK phosphorylation state in C3 leaves (Fig. 4). Similarly, rapid rates of PPDK dephosphorylation were observed when darkened (3 h) C3 leaves were exposed to light (≤15 min) and correspondingly slower rates of phosphorylation were observed when these leaves were returned to darkness (≥30–60 min; Fig. 4 [V. faba and F. pringlei]).
Figure 3.
Kinetic analysis of light-/dark-regulated changes in PPDK phosphorylation state in maize (C4) leaves. Each time point represents the relative image intensity of phospho-PPDK bands on immunoblots as a percent of that in a dark-adapted (2 h) leaf. A, Illumination of darkened leaves was initiated at approximately 800 μmol m−2 s−1 for a period of up to 15 min. B, After a 1-h illumination period, leaves were returned to darkness and sampled at 10 to 60 min. Soluble protein loaded per lane was 30 μg. Total dikinase protein per sample was normalized by probing duplicate blots with standard PPDK antibody. Each time point is the mean of three separate experiments. Inset in A shows standardized control blots of purified maize C4 PPDK showing linearity of scanned band-signal intensity up to 1 μg of PPDK. Bands on immunoblots used for Figures 3 and 4 were all within this linear range.
Figure 4.
Kinetic analysis of light-/dark-regulated changes in PPDK phosphorylation state in leaves of the C3 angiosperms F. pringlei (A) and V. faba (B). Each time point represents the relative image intensity of phospho-PPDK bands on immunoblots as a percent of that in a dark-adapted (3 h) leaf. Illumination of darkened leaves was initiated at approximately 800 μmol m−2 s−1 for a period of up to 60 min. After this time, the leaves were returned to darkness for further sampling. Load amount per lane was 100 μg of soluble protein concentrated from leaf extracts by fractionation with 35% to 55% saturation ammonium sulfate. Lanes were normalized for total PPDK by probing duplicate blots with standard PPDK antibody. Each time point in A or B is the mean of two or five separate experiments, respectively.
Light-/Dark-Induced Reversible Phosphorylation of PPDK in Isolated Intact Spinach Chloroplasts
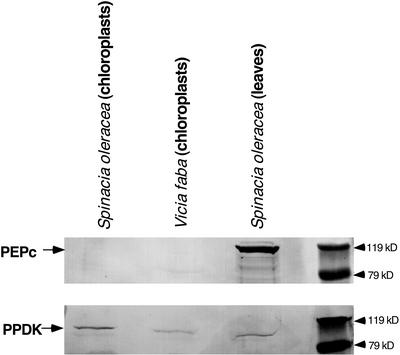
In C3 plants, PPDK is localized in the chloroplast and cytosol (see above). However, in C4 leaves, PPDK and RP are co-compartmentalized specifically in the mesophyll chloroplast stroma. Therefore, we assumed that the reversible phosphorylation of dikinase observed in various C3 leaves (Figs. 2 and 4) originated from the chloroplast-localized PPDK isoform. We directly investigated this possibility using an in organello approach for similar phospho-PPDK analyses of stromal extracts prepared from illuminated and dark-adapted intact spinach chloroplasts. Control immunoblot experiments with C3 chloroplast preparations isolated from both spinach and V. faba leaves documented the absence of cytosolic contamination of these plastids as evidenced by the lack of detectable PEPc protein, which is restricted to the cytosol in C3 and C4 plants (Fig. 5). In contrast, the stromal form of PPDK was readily observed in these isolated C3 chloroplasts. As we conjectured above, a light-/dark-induced reversible phosphorylation of C3 PPDK was observed in organello (Fig. 6). When intact chloroplasts isolated from darkened (1.5 h) spinach leaves (see control lanes) were illuminated, the rate of PPDK dephosphorylation was very rapid, with little or no phospho-PPDK detected after 10 min, similar to that observed in intact C3 leaves (Fig. 4).
Figure 5.
Cytosolic PEPc is undetectable in preparations of isolated intact V. faba and spinach chloroplasts. Duplicate immunoblots of spinach leaf and spinach and V. faba chloroplast soluble proteins, concentrated by 35% to 55% ammonium sulfate fractionation, were probed with maize PEPc antibody (above) or maize total PPDK antibody (below). Protein loads were 70 μg per lane. Arrows indicate the ≈110-kD PEPc monomer or ≈95-kD PPDK polypeptide.
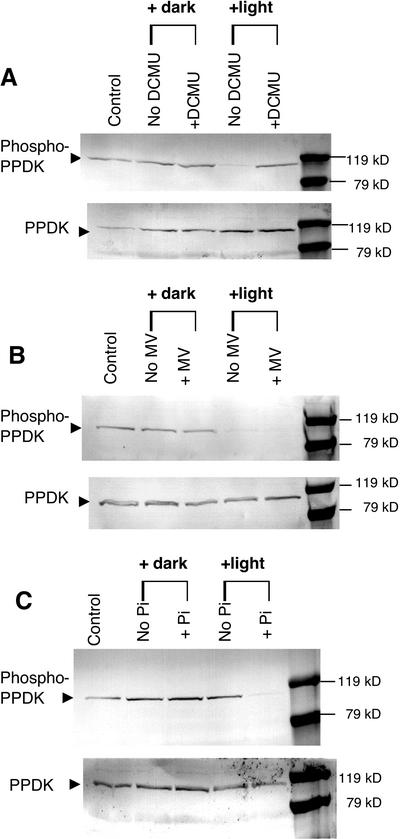
Figure 6.
Effects of a PSII inhibitor, a PSI alternate electron acceptor, and exogenous Pi on light-induced PPDK dephosphorylation in isolated intact spinach chloroplasts. Stromal extracts, fractionated with 35% to 55% saturation ammonium sulfate, were prepared from isolated intact chloroplasts incubated in the presence or absence of 20 μm 3-(3,4-dichlorophenyl)-1,1-dimethylurea (DCMU; A), 100 μm methyl viologen (MV; B), or 2 mm potassium Pi (C). Duplicate immunoblots were probed with either phospho-PPDK antibody (above) or standard PPDK antibody (below). Each lane contained 100 μg of stromal protein. Intact chloroplasts were darkened or illuminated at approximately 600 μmol m−2 s−1 for 10 min at 25°C before freezing in liquid N2. The lanes labeled as “control” represent the PPDK phosphorylation state before experimental manipulation of the intact chloroplasts isolated from the dark-adapted (1.5 h) parent leaves.
Effects of a PSII Inhibitor, a PSI Alternate Electron Acceptor, and Exogenous Pi on Light-/Dark-Induced Reversible Phosphorylation of PPDK in Isolated Intact Spinach Chloroplasts
One approach for gaining insight into the mechanism of light-/dark-modulated reversible phosphorylation of PPDK in C3 leaves and chloroplasts is to selectively manipulate PSII- and PSI-dependent electron transport during illumination of isolated intact spinach chloroplasts. Because the phosphorolytic dephosphorylation of C4 PPDK by RP is potently inhibited by ADP in vitro (Burnell and Hatch, 1985; Ashton et al., 1990), elevating stromal levels of ADP by inhibition of PSII, and thus non-cyclic photophosphorylation, would be expected to inhibit dephosphorylation of C3 phospho-PPDK in the light if the mechanism resembled that in C4 plants. When intact spinach chloroplasts were illuminated in the presence of the PSII inhibitor DCMU, light-induced dephosphorylation of phospho-PPDK was inhibited (Fig. 6A). In contrast, the artificial PSI terminal electron acceptor MV, which stimulates thylakoid ATP synthesis in the presence exogenous Pi (2 mm in these experiments) and conversely lowers stromal [ADP] (Robinson and Portis, 1988), had no effect on the light-induced dephosphorylation of PPDK (Fig. 6B). However, this treatment completely inhibited the thioredoxin-mediated light activation of stromal NADPH-malate dehydrogenase (data not shown), thus documenting that electron transfer after PSI was effectively negated in organello at its characterized site (i.e. reduction of oxidized ferredoxin and/or FA/FB clusters and NADP photoreduction; Malkin and Niyogi, 2000). Finally, we assessed the role of inorganic phosphate in the light-induced dephosphorylation mechanism because Pi is an actual substrate in the C4 RP-catalyzed dephosphorylation reaction (see Fig. 1). When exogenous Pi was omitted from the spinach chloroplast incubation medium during the 10-min illumination period, no dephosphorylation of stromal phospho-PPDK was observed (Fig. 6C).
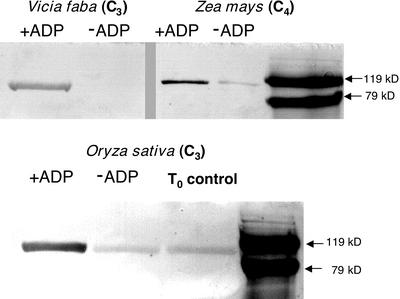
ADP-Dependent PPDK Phosphorylation Activity in C3 Leaf Extracts of Rice and V. faba
We employed an immunoblot-based in vitro assay, previously developed for measuring maize RP activity (Chastain et al., 2000), for detecting the presence of an RP-like activity in concentrated extracts prepared from V. faba and rice leaves. This assay is highly specific for RP because its basis is the regulatory phosphorylation of PPDK's “target” Thr residue with ADP as the specific phosphoryl donor. Using this method with desalted samples, a distinct band corresponding to phospho-PPDK was observed for both C3 species examined (+ADP lanes) that was either absent or much reduced when ADP was omitted from the reaction medium (−ADP lanes; Fig. 7). A parallel assay of a desalted maize leaf extract was performed as a control. The latter showed comparably less signal intensity because the quenched assay was diluted approximately 20-fold before loading onto SDS-PAGE gels versus the undiluted assay samples of the C3 extracts (Fig. 7).
Figure 7.
Purified maize PPDK is phosphorylated in vitro by an ADP-requiring activity present in desalted leaf extracts of V. faba and rice. The immunoblot-based, in vitro RP-phosphorylation assays were initiated by combining aliquots of desalted leaf extracts from maize, V. faba, or rice with purified maize recombinant (non-phospho) PPDK in the presence or absence of 1 mm ADP. Aliquots of the in vitro reactions, containing either 1.75 μg of PPDK with rice and V. faba or 90 ng for maize were electrophoresed in SDS-PAGE gels, and the resulting immunoblots probed with phospho-PPDK antibody. Visible bands indicate the position of phospho-PPDK corresponding to the ≈95-kD PPDK monomer as estimated by molecular mass standards on the same blot. The T0 control in the rice blot represents endogenous phospho-PPDK present in the dark-leaf extract before initiating the RP assay. Likewise, in the rice and maize −ADP lanes, the faint band of phospho-PPDK is the result of carryover of endogenous phospho-PPDK present in the aliquot of the dark-leaf extract used for the in vitro RP assay (Chastain et al., 2000).
DISCUSSION
Synthetic phosphopeptide-generated antibodies directed toward specific “target” proteins and phosphorylation sites have been used extensively for over a decade in animal cell biology for elucidating signaling pathways and protein kinase cascades but only recently and sparingly so in plants (Sugden et al., 1999; Chastain et al., 2000; Ueno et al., 2000). In this study, we further show the utility of such antibodies in plant research by characterizing the light/dark regulation of PPDK via reversible phosphorylation in leaves and isolated chloroplasts of C3 plants.
Early attempts at detecting reversible light activation of PPDK in C3 leaves were based on light/dark differences in extractable dikinase activity (Aoyagi and Bassham, 1984). These indirect studies were somewhat inconclusive because of the very low dikinase activity in C3 leaves and the intermingling of cytosolic and chloroplastic PPDK isoforms in whole tissue extracts, which may potentially diminish any light/dark differences in dikinase activity derived from the chloroplast fraction. More recently, however, Fukayama and coworkers (2001) were able to observe more measurable differences in extractable light/dark activity in transgenic rice leaves overexpressing maize C4 PPDK. These authors attributed the differences in extractable light/dark activity in their transgenic lines to an endogenous RP activity. However, with our more sensitive immunological approach, we could readily detect dark-/light-modulated phosphorylation of PPDK in C3 leaves and chloroplasts in a reasonably broad spectrum of species (Figs. 2, 4, and 6), and provide a more exacting characterization of the endogenous activation/inactivation mechanism. Further, we were also able to characterize this posttranslational modification with respect to subcellular location (chloroplasts) and kinetics (e.g. rate of phosphorylation/dephosphorylation during light/dark transitions). Together, these new findings indicate that light-/dark-induced reversible phosphorylation of C3 leaf PPDK is inherently similar to the same process occurring in C4 plants. For example, in maize, we found that light activation/dephosphorylation of PPDK is rapid and essentially complete in 8 min (Fig. 3A). The dark inactivation/phosphorylation process, however, is much slower, requiring approximately 1 h for maximal modification of the “target” enzyme (Fig. 3B). It is notable that a related experiment with the C3 species V. faba and F. pringlei (Asteraceae) demonstrated similarly different rates of light-mediated dephosphorylation and dark-induced phosphorylation (Fig. 4). Further, the finding that chloroplastic C3 PPDK undergoes light/dark changes in its phosphorylation state in organello (Fig. 6) signifies another similarity to the process in C4 leaves, where photosynthetic C4 PPDK is reversibly phosphorylated in chloroplasts of mesophyll cells. We hypothesize that it is highly unlikely that the cytosol-localized C3 PPDK isoform also undergoes reversible phosphorylation at the same active-site Thr.
Extensive in vitro studies with C4 RP demonstrate that the basis for the light/dark regulation mechanism is likely fluctuating ADP and Pi levels in the stroma (see discussion below). In the C3 cytosol, these metabolites show little diurnal variation (Stitt et al., 1982) and so the necessary light/dark regulatory signals for PPDK inactivation/activation are lacking. Because of these striking similarities to the reversible phosphorylation of C4 PPDK (Figs. 2–4 and 6), we conjectured that a C3 precursor to RP, or an RP-like activity, might be catalyzing this modification of dikinase in C3 chloroplasts. We addressed this possibility by manipulating stromal levels of ADP, a potent in vitro metabolite regulator of C4 RP activity (Burnell and Hatch, 1985; Ashton et al., 1990), via selective perturbation of PSII and PSI electron transfer in intact spinach chloroplasts. Although C4 RP has not been reproducibly purified to homogeneity (compare Burnell and Hatch, 1985; Roeske and Chollet, 1987; Smith et al., 1994b) and therefore no related antibodies or gene clones presently exist, studies of the partially purified enzyme have elucidated a general reaction mechanism (Fig. 1; Burnell and Hatch, 1985; Roeske and Chollet, 1989; Ashton et al., 1990). These same studies have not yielded a conclusive understanding of exactly how its opposing protein kinase and phosphatase activities are regulated, but a proposed mechanism involves the balance of competing substrates for RP (primarily ADP and Pi) in the chloroplast stroma (Burnell and Hatch, 1985; Roeske and Chollet, 1989; Ashton et al., 1990, Malkin and Niyogi, 2000). According to this working model, lower levels of stromal ADP in the light favor the reactivation/dephosphorylation (PPi-forming) reaction. Conversely, in the dark, higher ADP levels favor the inactivation/phosphorylation process.
Moreover, ADP is also a potent competitive inhibitor of the dephosphorylation reaction in vitro with respect to the inactive, PPDK-ThrP enzyme form (Burnell and Hatch, 1985; Roeske and Chollet, 1989; Ashton et al., 1990). When spinach chloroplasts were illuminated in the presence of the PSII inhibitor DCMU, light-induced dephosphorylation of phospho-PPDK was inhibited (Fig. 6A). This observation is consistent with the proposed C4 RP reaction/regulatory mechanism in that DCMU dramatically decreases non-cyclic photophosphorylation, thereby resulting in elevated ADP levels (Malkin and Niyogi, 2000). Supporting this contention are earlier studies that examined the effects of DCMU and an uncoupler of photophosphorylation, carbonyl cyanide m-chlorophenylhydrazone, on maize C4 mesophyll protoplast and chloroplast PPDK activity. These investigators found that illuminating the mesophyll preparations in the presence of DCMU or m-chlorophenylhydrazone markedly inhibited light activation of PPDK and this was correlated with lowered stromal ATP concentrations in the light (Nakamoto and Edwards, 1986; Nakamoto and Young, 1990). Conversely, when spinach chloroplasts were illuminated in the presence of MV, an artificial PSI terminal electron acceptor, no inhibition of PPDK dephosphorylation was observed (Fig. 6B). This also is consistent with the C4 RP reaction/regulatory mechanism because MV has been shown to stimulate C3 chloroplast ATP production and hence decrease stromal ADP levels via photophosphorylation (Robinson and Portis, 1988). Further, because the site of action of MV prevents photosynthetic reduction of stromal thioredoxin and NADP, reversible phosphorylation of PPDK in C3 leaves appears neither to be redox regulated, which is consistent with previous studies concerning C4 RP (Yamamoto et al., 1974; Nakamoto and Young, 1990; Smith et al., 1994b), nor dependent on Calvin cycle activity.
Additional evidence supporting our view of a chloroplastic form of C3 RP is the finding that the light-induced dephosphorylation of phospho-PPDK in isolated spinach chloroplasts is Pi dependent (Fig. 6C). The dephosphorylation reaction catalyzed by RP is highly unusual in that it is a phosphorolytic process rather than simply hydrolytic as in most protein phosphatases. Hence, Pi deprivation during illumination of isolated C3 chloroplasts may prevent dephosphorylation by limiting substrate for the RP-catalyzed reactivation (dephosphorylation) reaction. Alternatively, decreased stromal [Pi] would impair ATP synthesis in the light (Robinson and Portis, 1988) and thereby result in increased ADP levels. Thus, the inhibitory effect of Pi deprivation on the dephosphorylation of C3 chloroplast PPDK (Fig. 6C) may be a synergistic combination of elevated ADP and decreased Pi levels in the stroma.
Perhaps the most direct evidence for a C3 RP is the in vitro demonstration of an ADP-dependent PPDK phosphorylation activity present in desalted crude leaf extracts of V. faba and rice (Fig. 7). The highly specific nature of this immunological assay would preclude any other “promiscuous” kinase activity from producing the results we observed. First, the specific phosphoryl donor in the reaction is ADP, an unprecedented substrate for eukaryotic protein kinases (Hanks and Hunter, 1995; Hardie, 1999). Second, the phospho-PPDK antibody used in these assays detects only PPDK phosphorylated at the active-site “target” Thr residue (Chastain et al., 2000).
CONCLUSIONS
That RP or an RP-like activity is present in C3 leaves and chloroplasts should not be surprising. The independent emergence of C4 photosynthesis in diverse angiosperm families implies a more or less common evolutionary pathway. Supporting this notion is the fact that all of the C4 cycle enzymes occur in C3 plants, albeit functioning as non-photosynthetic isoforms (Edwards et al., 2001). Less obvious for this hypothesis has been the C3 origins of proteins that up-/down-regulate certain “target” enzymes in the C4 pathway. As discussed above, PpcK and PEPc phosphatase, the opposing C4 PEPc regulatory enzymes, are now known to function in the reversible phosphorylation of non-photosynthetic PEPc in C3 plants (Duff and Chollet, 1995; Zhang et al., 1995; Li et al., 1996). Based on the evidence gathered in this study, we propose that a C3 precursor to C4 RP, or a related RP-like activity, is present in chloroplasts of C3 plants and functions similarly in the light/dark regulation of C3 PPDK activity. The documentation of these regulatory enzyme activities in C3 leaves lends major support for the hypothesis of a common evolutionary pathway to account for the multiple emergence of C4 photosynthesis in angiosperms. Another implication of our findings pertains to the possible function(s) of PPDK in C3 leaves. The discovery of a light-regulated PPDK in C3 chloroplasts implies that de novo PEP synthesis in the stroma is necessarily linked to the availability of light energy, and so should yield insight into a possible function for C3 dikinase. For example, one function may be to supplement the stromal pool of PEP normally imported from the cytosol via the plastidic PEP/Pi translocator (Flügge, 1999). The relevance of a mechanism for augmenting the PEP pool in chloroplasts may be related to the requirement for PEP as a precursor for aromatic amino acid biosynthesis initiated by the chloroplastic shikimic acid pathway (Coruzzi and Last, 2000).
MATERIALS AND METHODS
Plant Material
All plants were greenhouse grown with supplemental lighting under standard cultural practices. Plants were watered daily and fertilized twice a week with nutrient solution (N:P:K, 20:20:20 [w/w], Peters Professional, Allentown, PA). The maize (Zea mays cv Golden Bantam) and rice (Oryza sativa cv Jackson) seedlings utilized in this study were grown to the 4- to 6-week-old stage. Spinach (Spinacia oleracea cv Bloomsdale Longstanding), Flaveria pringlei, and Vicia faba plants were grown to the 8-week-old stage.
Immunoblot Analysis of PPDK, Phospho-PPDK, and PEPc
Detached whole leaves (V. faba, spinach, and F. pringlei) or 4-cm leaf segments (maize and rice) were floated on distilled water at 25°C in a 20-cm-diameter glass dish and dark adapted before illumination as described in the figure legends. For illumination, a halogen lamp was positioned above the dish with a 10-cm layer of water between the lamp and the floating leaves to minimize excess heat. The photosynthetic photon flux density at the leaf surface was approximately 800 μmol m−2 s−1. After each light or dark treatment, leaves were removed and flash frozen in liquid N2 and subsequently stored at −80°C for a period of up to 4 weeks before extraction.
To obtain soluble protein samples for immunoblot analyses, the frozen C3 leaves (0.5–1.0 g) were ground to a fine powder in a mortar and pestle with dry ice and homogenized in 4 mL of extraction buffer (50 mm Tris-HCl, pH 8.0; 2 mm EDTA; 2 μm orthovanadate; 1 mm phenylmethylsulfonyl fluoride; 0.01% [v/v] protease inhibitor cocktail [Sigma, St. Louis], and 1% [w/v] insoluble polyvinylpyrrolidone). The homogenate was clarified by a 10-min centrifugation at 48,000g and 4°C and then subjected to fractionation with 35% to 55% saturation ammonium sulfate. For SDS-PAGE, the resulting pellet was resuspended in 0.5 mL of extraction buffer and desalted by buffer exchange using centrifugal filtration. Aliquots of this concentrated, desalted protein sample were combined with SDS-sample buffer and loaded directly onto 10% (w/v) SDS-PAGE gels for electrophoresis. For maize, the leaves were homogenized as above, and the crude extract clarified by a 3-min, 14,000g centrifugation and used directly for immunoblot analysis. After electrophoretic transfer to nitrocellulose membrane, blots were hybridized with primary and secondary antibodies using standard techniques (Ausubel et al., 1999). The primary antibodies used in this study were: (a) affinity-purified rabbit polyclonal antibodies raised against a synthetic phosphopeptide conjugate corresponding to the Thr-phosphorylation domain of maize C4 PPDK (residues 445–464 [AVGILTERGGMTpSHAAVVAR]; Chastain et al., 2000), (b) rabbit polyclonal antibodies raised against the maize PPDK monomer (Budde and Chollet, 1986), and (c) rabbit polyclonal antibodies raised against the maize PEPc monomer (Budde and Chollet, 1986).
Detection of the respective antigen/antibody complexes on immunoblots was accomplished using the chromogenic alkaline phosphatase substrate nitroblue tetrazolium/ 5-bromo-4-chloro-3-indolyl phosphate (Endogen 1-Step nitroblue tetrazolium/5-bromo-4-chloro-3-indolyl phosphate, Pierce Chemical, Rockford, IL). Use of immunoblot analysis for assessing the relative phosphorylation state of PPDK during light/dark transitions was accomplished by scanning the developed blots on a flat-bed scanner. Quantitative estimates of band optical density were obtained using the Multi-Analyst software package (Bio-Rad Laboratories, Hercules, CA). Phospho-PPDK bands were normalized with respect to total PPDK per lane by probing duplicate blots with standard PPDK antibody. The scanning methodology employed for this study was shown to be linear for lane loads of 0.05 to 1 μg total PPDK per band (see inset in Fig. 3A).
Isolation of Intact C3 Chloroplasts and Light/Dark Adaptation Studies
Intact spinach and V. faba chloroplasts were isolated using a previously developed protocol, except that before leaf harvest the plants were dark adapted for 1.5 h at room temperature (Robinson and Portis, 1988). The Percoll-gradient purified, intact chloroplast pellet was resuspended in 0.33 m sorbitol; 50 mm HEPES-KOH, pH 7.6; 2 mm EDTA; 1 mm MgCl2; 1 mm MnCl2; and 0.2% (w/v) bovine serum albumin (BSA) to a final concentration of approximately 150 μg chlorophyll mL−1 and stored on ice until use. Three milliliters of the chloroplast suspension was placed into a 15-mL, clear conical centrifuge tube containing 2 mm Pi (K+ salt), 1 mm KHCO3, and 700 units mL−1 catalase, unless noted otherwise. Experiments were initiated by placing the tube containing a microstir bar into a 25°C Plexiglas water bath with gentle stirring from beneath. Chloroplasts were illuminated from the side using a halogen lamp at a photosynthetic photon flux density of approximately 600 μmol m−2 s−1. After a 10-min period of illumination or darkness, the tubes were plunged into liquid N2 and stored at −80°C before extraction. Protein aliquots for immunoblot analysis were obtained from the frozen chloroplasts by thawing the suspension in two volumes of lysis buffer (50 mm Tris-HCl, pH 8.0; 2 mm EDTA; 2 μm orthovanadate; 1 mm phenylmethylsulfonyl fluoride; 0.01% [v/v] protease inhibitor cocktail [Sigma]; and 0.05% [v/v] Triton X-100). The centrifuged chloroplast lysate was then fractionated with 35% to 55% saturation ammonium sulfate, and the resulting protein pellet was resuspended and desalted for SDS-PAGE and subsequent immunoblotting as described above.
In Vitro Assays of RP and NADPH-Malate Dehydrogenase Activity
The source of PPDK-RP for these experiments was a “rapid” leaf extract prepared as described previously from dark-adapted leaf tissue (Smith et al., 1994a). Phosphorylation assays were composed of 50 mm Bicine-KOH, pH 8.3; 10 mm MgCl2; 5 mm dithiothreitol; 1 mg mL−1 BSA; 1 mm ADP; 0.2 mm ATP; and 35 μg of affinity-purified, maize recombinant C4 PPDK (non-phospho form; previously heat activated by pre-incubation at 30°C for 10 min; Chastain et al., 1997). Immediately after desalting, 10 μL (approximately 30 μg protein) of the “rapid” leaf extract was added to the reaction medium to bring the final volume to 100 μL, and the assay incubated at 30°C for 30 min. The reactions were terminated by addition of an equal volume of SDS-sample buffer followed by heating at 100°C for 3 min. Aliquots of the quenched assays were loaded onto 10% (w/v) SDS-polyacrylamide gels and electrophoresed. The gels were electroblotted onto nitrocellulose membrane and probed with phospho-PPDK peptide antibody as described above.
Spinach chloroplast NADPH-malate dehydrogenase activity was assayed spectrophotometrically at 340 nm, according to a previously published method (Nakamoto and Edwards, 1983).
Protein and Chlorophyll Assays
Protein was quantified by a dye-binding method with crystalline BSA as standard (Bradford, 1976). Chlorophyll was determined according to the method of Arnon (1949).
Distribution of Materials
Upon request, all novel materials described in this publication will be made available in a timely manner for noncommercial research purposes.
Footnotes
This work was supported in part by the U.S. National Science Foundation (grant nos. RUI–0094497 to C.J.C. and MCB–9727236 to R.C.) and by the Center for Biotechnology, University of Nebraska, Lincoln, funded through the Nebraska Research Initiative (to G.S.).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.010806
LITERATURE CITED
- Agarie S, Kai M, Takatsuji H, Ueno O. Expression of C3 and C4 photosynthetic characteristics in the amphibious plant Eleocharis vivipara: structure and analysis of the expression of isogenes for pyruvate,orthophosphate dikinase. Plant Mol Biol. 1997;34:363–369. doi: 10.1023/a:1005897118660. [DOI] [PubMed] [Google Scholar]
- Aoyagi K, Bassham JA. Pyruvate orthophosphate dikinase of C3 seeds and leaves as compared to the enzyme from maize. Plant Physiol. 1984;75:387–392. doi: 10.1104/pp.75.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoyagi K, Bassham JA. Synthesis and uptake of cytoplasmically synthesized pyruvate, Pi dikinase polypeptide by chloroplasts. Plant Physiol. 1985;78:807–811. doi: 10.1104/pp.78.4.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoyagi K, Chua N-H. Cell-specific expression of pyruvate, Pi dikinase: in situ mRNA hybridization and immunolocalization labeling of protein in wheat seed. Plant Physiol. 1988;86:364–368. doi: 10.1104/pp.86.2.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnon DI. Copper enzymes in isolated chloroplasts: polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949;24:1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashton AR, Burnell JN, Furbank RT, Jenkins CLD, Hatch MD. Enzymes of C4 photosynthesis. In: Lea PJ, editor. Methods in Plant Biochemistry. Vol. 3. San Diego: Academic Press; 1990. pp. 39–72. [Google Scholar]
- Ausubel FM, Kingstown BR, Moore DD, Seidman JG, Smith JA, Struhl K, editors. Current Protocols in Molecular Biology. New York: John Wiley & Sons; 1999. [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Budde RJA, Chollet R. In vitro phosphorylation of maize leaf phosphoenolpyruvate carboxylase. Plant Physiol. 1986;82:1107–1114. doi: 10.1104/pp.82.4.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnell JN. A comparative study of the cold-sensitivity of pyruvate, Pi dikinase in Flaveria species. Plant Cell Physiol. 1990;31:295–297. [Google Scholar]
- Burnell JN, Hatch MD. Regulation of C4 photosynthesis: identification of a catalytically important histidine residue and its role in the regulation of pyruvate,Pi dikinase. Arch Biochem Biophys. 1984;231:175–182. doi: 10.1016/0003-9861(84)90375-8. [DOI] [PubMed] [Google Scholar]
- Burnell JN, Hatch MD. Regulation of C4 photosynthesis: purification and properties of the protein catalyzing ADP-mediated inactivation and Pi-mediated activation of pyruvate, Pi dikinase. Arch Biochem Biophys. 1985;237:490–503. doi: 10.1016/0003-9861(85)90302-9. [DOI] [PubMed] [Google Scholar]
- Burnell JN, Hatch MD. Activation and inactivation of an enzyme catalyzed by a single, bifunctional protein: a new example and why. Arch Biochem Biophys. 1986;245:297–304. doi: 10.1016/0003-9861(86)90219-5. [DOI] [PubMed] [Google Scholar]
- Chastain CJ, Botschner M, Harrington GS, Thompson BJ, Mills SE, Sarath G, Chollet R. Further analysis of maize C4-pyruvate,orthophosphate dikinase phosphorylation by its bifunctional regulatory protein using selective substitutions of the regulatory Thr-456 and catalytic His-458 residues. Arch Biochem Biophys. 2000;375:165–170. doi: 10.1006/abbi.1999.1651. [DOI] [PubMed] [Google Scholar]
- Chastain CJ, Lee ME, Moorman MA, Shameekumar P, Chollet R. Site-directed mutagenesis of maize recombinant C4-pyruvate,orthophosphate dikinase at the phosphorylatable target threonine residue. FEBS Lett. 1997;413:169–173. doi: 10.1016/s0014-5793(97)00884-3. [DOI] [PubMed] [Google Scholar]
- Coruzzi G, Last R. Amino Acids. In: Buchanan BB, Gruissem W, Jones RL, editors. Biochemistry and Molecular Biology of Plants. Rockville, MD: American Society of Plant Physiologists; 2000. pp. 358–410. [Google Scholar]
- Duff SMG, Chollet R. In vivo regulation of wheat-leaf phosphoenolpyruvate carboxylase by reversible phosphorylation. Plant Physiol. 1995;107:775–782. doi: 10.1104/pp.107.3.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards GE, Furbank RT, Hatch MD, Osmond CB. What does it take to be C4? Lessons from the evolution of C4 photosynthesis. Plant Physiol. 2001;125:46–49. doi: 10.1104/pp.125.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisslthaler B, Meyer G, Bohnert HJ, Schmitt JM. Age-dependent expression of pyruvate,orthophosphate dikinase in Mesembryanthemum crystallinum L. Planta. 1995;196:492–500. doi: 10.1007/BF00203649. [DOI] [PubMed] [Google Scholar]
- Flügge U-I. Phosphate translocators in plastids. Annu Rev Plant Physiol Plant Mol Biol. 1999;50:27–45. doi: 10.1146/annurev.arplant.50.1.27. [DOI] [PubMed] [Google Scholar]
- Fukayama H, Tsuchida H, Agarie S, Nomura M, Onodera H, Ono K, Lee B-H, Hirose S, Toki S, Ku MSB et al. Significant accumulation of C4-specific pyruvateorthophosphate dikinase in a C3 plant, rice. Plant Phsyiol. 2001;127:1136–1146. [PMC free article] [PubMed] [Google Scholar]
- Furbank RT, Chitty JA, Jenkins CLD, Taylor WC, Trevanion SJ, Caemmerer SV, Ashton AR. Genetic manipulation of key photosynthetic enzymes in the C4 plant Flaveria bidentis. Aust J Plant Physiol. 1997;24:477–485. [Google Scholar]
- Gallusci P, Varotto S, Matsuoka M, Maddaloni M, Thompson MD. Regulation of cytosolic pyruvate, orthophosphate dikinase expression in developing maize endosperm. Plant Mol Biol. 1996;31:45–55. doi: 10.1007/BF00020605. [DOI] [PubMed] [Google Scholar]
- Hanks SK, Hunter T. The eukaryotic protein kinase super family: kinase (catalytic) domain structure and classification. FASEB J. 1995;9:576–596. [PubMed] [Google Scholar]
- Hardie DG. Plant protein serine/threonine kinases: classification and functions. Annu Rev Plant Physiol Plant Mol Biol. 1999;50:97–131. doi: 10.1146/annurev.arplant.50.1.97. [DOI] [PubMed] [Google Scholar]
- Hata S, Matsuoka M. Immunological studies on pyruvate,orthophosphate dikinase in C3 plants. Plant Cell Physiol. 1987;28:635–641. [Google Scholar]
- Hatch MD. C4 photosynthesis: a unique blend of modified biochemistry, anatomy and ultrastructure. Biochim Biophys Acta. 1987;895:81–106. [Google Scholar]
- Hocking CG, Anderson JW. Survey of pyruvate, phosphate dikinase activity of plants in relation to C3, C4 and CAM mechanisms of CO2 assimilation. Phytochemistry. 1986;25:1537–1543. [Google Scholar]
- Imaizumi N, Ku MSB, Ishihara K, Samejima M, Kaneko S, Matsuoka M. Characterization of the gene for pyruvate,orthophosphate dikinase from rice, a C3 plant, and comparison of structure and expression between C3 and C4 genes for this protein. Plant Mol Biol. 1997;34:701–716. doi: 10.1023/a:1005884515840. [DOI] [PubMed] [Google Scholar]
- Li B, Zhang X-Q, Chollet R. Phosphoenolpyruvate carboxylase kinase in tobacco leaves is activated by light in a similar but not identical way as in maize. Plant Physiol. 1996;111:497–505. doi: 10.1104/pp.111.2.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malkin R, Niyogi K. Photosynthesis. In: Buchanan BB, Gruissem W, Jones RL, editors. Biochemistry and Molecular Biology of Plants. Rockville, MD: American Society of Plant Physiologists; 2000. pp. 568–628. [Google Scholar]
- Moons A, Valcke R, Van Montagu M. Low-oxygen stress and water deficit induce cytosolic pyruvate orthophosphate dikinase (PPDK) expression in roots of rice, a C3 plant. Plant J. 1998;15:89–98. doi: 10.1046/j.1365-313x.1998.00185.x. [DOI] [PubMed] [Google Scholar]
- Nakamoto H, Edwards GE. Influence of oxygen and temperature on the dark inactivation of pyruvate,orthophosphate dikinase and NADP-malate dehydrogenase in maize. Plant Physiol. 1983;71:568–573. doi: 10.1104/pp.71.3.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamoto H, Edwards GE. Light activation of pyruvate,Pi dikinase and NADP-malate dehydrogenase in mesophyll protoplasts of maize: effect of DCMU, antimycin A, CCCP, and phlorizin. Plant Physiol. 1986;82:312–315. doi: 10.1104/pp.82.1.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamoto H, Young PS. Light activation of pyruvate, orthophosphate dikinase in maize mesophyll chloroplasts: a role of adenylate energy charge. Plant Cell Physiol. 1990;31:1–6. [Google Scholar]
- Nomura M, Sentoku N, Tajima S, Matsuoka M. Expression patterns of cytoplasmic pyruvate,orthophosphate dikinase of rice (C3) and maize (C4) in a C3 plant, rice. Aust J Plant Physiol. 2000;27:343–347. [Google Scholar]
- Robinson SP, Portis AR. Involvement of stromal ATP in the light activation of ribulose-1,5-bisphosphate carboxylase/oxygenase in intact isolated chloroplasts. Plant Physiol. 1988;86:293–298. doi: 10.1104/pp.86.1.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roeske CA, Chollet R. Chemical modification of the bifunctional regulatory protein of maize leaf pyruvate, orthophosphate dikinase: evidence for two distinct active sites. J Biol Chem. 1987;262:12575–12582. [PubMed] [Google Scholar]
- Roeske CA, Chollet R. Role of metabolites in the reversible light activation of pyruvate,orthophosphate dikinase in Zea mays mesophyll cells in vivo. Plant Physiol. 1989;90:330–337. doi: 10.1104/pp.90.1.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosche E, Streubel M, Westhoff P. Primary structure of the pyruvate orthophosphate dikinase of the C3 plant Flaveria pringlei and expression analysis of pyruvate orthophosphate dikinase sequences in C3, C3-C4 and C4 Flaveria species. Plant Mol Biol. 1994;26:763–769. doi: 10.1007/BF00013761. [DOI] [PubMed] [Google Scholar]
- Smith CM, Sarath G, Chollet R. A simple, single-tube radioisotopic assay for the phosphorylation/inactivation activity of the pyruvate,orthophosphate dikinase regulatory protein. Photosynth Res. 1994a;40:295–301. doi: 10.1007/BF00034779. [DOI] [PubMed] [Google Scholar]
- Smith CM, Duff SMG, Chollet R. Partial purification and characterization of maize-leaf pyruvate, orthophosphate dikinase regulatory protein: a low-abundance, mesophyll-chloroplast stromal protein. Arch Biochem Biophys. 1994b;308:200–206. doi: 10.1006/abbi.1994.1028. [DOI] [PubMed] [Google Scholar]
- Smith RD, Walker JC. Plant protein phosphatases. Annu Rev Plant Physiol Plant Mol Biol. 1996;47:101–125. doi: 10.1146/annurev.arplant.47.1.101. [DOI] [PubMed] [Google Scholar]
- Stitt M, McLilley R, Heldt HW. Adenine nucleotide levels in the cytosol, chloroplasts, and mitochondria of wheat leaf protoplasts. Plant Physiol. 1982;70:971–977. doi: 10.1104/pp.70.4.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugden C, Crawford RM, Halford NG, Hardie DG. Regulation of spinach SNF1-related kinases by protein kinases and phosphatases is associated with phosphorylation of the T loop and is regulated by 5′-AMP. Plant J. 1999;19:433–439. doi: 10.1046/j.1365-313x.1999.00532.x. [DOI] [PubMed] [Google Scholar]
- Ueno Y, Imanari E, Emura J, Yoshizawa-Kumagaye K, Nakajima K, Inami K, Shiba T, Sakakibara H, Sugiyama T, Izui K. Immunological analysis of the phosphorylation state of maize C4-form phosphoenolpyruvate carboxylase with specific antibodies raised against a synthetic phosphorylated peptide. Plant J. 2000;21:17–26. doi: 10.1046/j.1365-313x.2000.00649.x. [DOI] [PubMed] [Google Scholar]
- Vidal J, Chollet R. Regulatory phosphorylation of C4 PEP carboxylase. Trends Plant Sci. 1997;2:230–237. [Google Scholar]
- Wei M, Li Z, Ye D, Herzberg O, Dunaway-Mariano D. Identification of domain-docking sites within Clostridium symbiosum pyruvate phosphate dikinase by amino acid replacement. J Biol Chem. 2000;275:41156–41165. doi: 10.1074/jbc.M006149200. [DOI] [PubMed] [Google Scholar]
- Yamamoto E, Sugiyama T, Miyachi S. Action spectrum for light activation of pyruvate,phosphate dikinase in maize leaves. Plant Cell Physiol. 1974;15:987–992. [Google Scholar]
- Zhang X-Q, Li B, Chollet R. In vivo regulatory phosphorylation of soybean nodule phosphoenolpyruvate carboxylase. Plant Physiol. 1995;108:1561–1568. doi: 10.1104/pp.108.4.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]