Abstract
Growth of young pea (Pisum sativum) fruit (pericarp) requires developing seeds or, in the absence of seeds, treatment with gibberellin (GA) or auxin (4-chloroindole-3-acetic acid). This study examined the role of seeds and hormones in the regulation of cell division and elongation in early pea fruit development. Profiling histone H2A and γ-tonoplast intrinsic protein (TIP) gene expression during early fruit development identified the relative contributions of cell division and elongation to fruit growth, whereas histological studies identified specific zones of cell division and elongation in exocarp, mesocarp, and endocarp tissues. Molecular and histological studies showed that maximal cell division was from −2 to 2 d after anthesis (DAA) and elongation from 2 to 5 DAA in pea pericarp. Maximal increase in pericarp γ-TIP message level preceded the maximal rate of fruit growth and, in general, γ-TIP mRNA level was useful as a qualitative marker for expanding tissue, but not as a quantitative marker for cell expansion. Seed removal resulted in rapid decreases in pericarp growth and in γ-TIP and histone H2A message levels. In general, GA and 4-chloroindole-3-acetic acid maintained these processes in deseeded pericarp similarly to pericarps with seeds, and both hormones were required to obtain mesocarp cell sizes equivalent to intact fruit. However, GA treatment to deseeded pericarps resulted in elevated levels of γ-TIP mRNA (6 and 7 DAA) when pericarp growth and cell enlargement were minimal. Our data support the theory that cell division and elongation are developmentally regulated during early pea fruit growth and are maintained by the hormonal interaction of GA and auxin.
Fruit development involves a complex interaction of molecular, biochemical, and structural changes that transform a fertilized ovary into a mature fruit. The functions of the ovary (pericarp) are to protect the developing seeds against mechanical damage, to stabilize the micro-environment during seed ontogeny, and to act as a physiological buffer against fluctuations in the nutrient supply (Müntz et al., 1978). To carry out these functions, communication between the developing seeds and the pericarp is required. Fruit development in pea (Pisum sativum) has been characterized physiologically and biochemically (Sutcliffe and Pate, 1977; Hebblethwaite et al., 1985) and represents a useful system to learn more about complex regulatory mechanisms that control the division, growth, and differentiation of plant cells. However, little information is available on the physiology of early fruit development, on the molecular aspects of fruit development in general, and on how fruit development is coordinated with seed formation. Fruit set and sustained pod elongation in pea are normally dependent on the presence of seeds. It is likely that the developing seeds produce signal molecules that regulate cellular division and expansion of the surrounding fruit tissues. Gibberellins (GAs; biologically active GA1 and GA3; Garcia-Martinez et al., 1991; Rodrigo et al., 1997) and auxins (4-chloroindole-3-acetic acid [4-Cl-IAA] and IAA; Marumo et al., 1968; Magnus et al., 1997) are natural constituents of pea seeds and pericarps and are likely candidates for such signal molecules. In pea, it has been assumed that GAs biosynthesized by the seeds are transported to the pericarp and regulate pericarp growth (Garcia-Martinez et al., 1991; Rodrigo et al., 1997). However, genetic evidence (young seeds of the pea GA biosynthesis mutant ls-1 contain relatively normal levels of GA1, but the pericarps are GA1-deficient; MacKenzie-Hose et al., 1998) suggests that it is unlikely that significant GA transport occurs from the seed to the pericarp. An alternative hypothesis that seeds may promote pericarp growth by maintaining pericarp GA biosynthesis has been proposed (Sponsel, 1982). Results obtained using a split-pericarp system suggest that upon seed removal, a key step in the GA biosynthesis pathway is inhibited (conversion of GA19 to GA20; Ozga et al., 1992). The natural pea auxin 4-Cl-IAA can substitute for the seeds in the stimulation of growth (Reinecke et al., 1995), conversion of GA19 to GA20 (van Huizen et al., 1995), and GA 20-oxidase gene expression (GA 20-oxidase genes code for the enzyme that converts GA19 to GA20; van Huizen et al., 1997) in pea pericarp. In previous in vivo protein synthesis studies (van Huizen et al., 1996), the application of 4-Cl-IAA plus GA3 mimicked the seed effect on protein synthesis in the pericarp. The GA 20-oxidase gene expression and in vivo protein synthesis studies support the hypothesis that both hormones are involved in pea pericarp development. However, GA 20-oxidase gene expression and polypeptide synthesis patterns unique to GA3 or 4-Cl-IAA treatment also indicate that their effects on these processes are not equivalent.
Auxin and GAs have long been acknowledged as regulators of cellular division and elongation (Davies, 1995). Auxin (4-Cl-IAA) and GA stimulate deseeded pea pericarp growth (length and fresh weight) and, together, synergistically enhance growth (Ozga and Reinecke, 1999). 4-Cl-IAA is very active in auxin tissue bioassays (see Reinecke et al., 1995), but how 4-Cl-IAA and 4-Cl-IAA plus GA effect growth at the cellular level is not known. The objectives of this study were to characterize cellular division and expansion activities during early pea fruit development and to determine how hormonal signals alter these growth parameters.
The availability of plant cell cycle-dependent genes provides a means for examining the pattern of mitotic activity during early fruit development and the influence of plant growth regulators on this process. The regulation of replication-dependent histone expression has been extensively studied (for review, see Osley, 1991). Tanimoto et al. (1993) found that histone H2A mRNA transiently accumulated in apical meristems of pea root tips during a period of the cell cycle that mostly overlapped the S phase. In tomato, Koning et al. (1991) found that the steady-state histone H2A message was abundant in cycling cells like apices and early developing fruit and was very low in mature tissue. Therefore, expression of this histone is replication dependent, and accumulation of its mRNA is useful as a marker for cell division as long as endoduplication in the tissue of interest is minimal (Koning et al., 1991).
Before the cell enlargement phase, cells in the developing pea fruits are small, and tightly compressed. As cells enlarge, the vacuoles occupy a greater proportion of the cell volume (Vercher et al., 1984). Parameters determining final cell size are cell wall extensibility, solute accumulation, and water uptake (Lockhart, 1965). Aquaporins define a functional class of water-transport proteins that belong to the larger major intrinsic protein family of transmembrane channels (see Maurel, 1997). γ-TIPs (tonoplast intrinsic proteins) are a subclass of aquaporins that are capable of forming transmembrane channels that allow the passive transfer of water into the tonoplast (Maurel et al., 1993) and are differentially expressed in organs or as a result of specific signals (Ludevid et al., 1992; Maurel, 1997). Ludevid et al. (1992) examined the expression of γ-TIP in Arabidopsis using γ-TIP promoter-β-glucuronidase (GUS) fusions and in situ hybridization. They found that γ-TIP expression followed a transient pattern of gene expression that was associated with cell expansion (when large central vacuoles are being formed) in the hypocotyls, petioles, and roots of 2- to 5 d-old seedlings. No GUS activity was detected in the very young ovaries from transformed plants with the γ-TIP-GUS fusion; however, GUS activity was detected in later development of the silique (but no data was presented). γ-TIP expression also increased during GA3-induced stem elongation in the Arabidopsis ga1 mutant (which has very low levels of endogenous active GAs; Phillips and Huttly, 1994). Because γ-TIP mRNA is primarily expressed in rapidly expanding tissues, the gene product is thought to be involved in facilitating rapid water influx into enlarging vacuoles during cellular expansion. The degree to which γ-TIP expression is correlated with cellular expansion is not known.
In this study, histone H2A and γ-TIP gene expression was used to characterize growth of plant tissue (pea pericarp) that is responsive to both GAs and auxin (4-Cl-IAA). Specifically, the relationship between increase in cell number and histone H2A gene expression and cellular enlargement and γ-TIP gene expression was examined during seed- and hormone-induced pea fruit growth. Our results suggest that GAs and auxins can replace the requirement of seeds for maintenance of cellular division and elongation processes during early fruit development and that both hormones are required to obtain cell size equivalent to intact fruit. We found that histone H2A gene expression closely followed mitotic activity in the pericarp. In addition, γ-TIP mRNA levels correlated with pericarp growth rate, but this correlation was not absolute. Our data support the theory that γ-TIP is involved in cell expansion in pericarp tissue along with other factors that may limit growth under some conditions.
RESULTS
GA3- and 4-Cl-IAA-Stimulated Pericarp Growth
Splitting of the pericarp 2 d after anthesis (DAA) without disturbing the seeds (SP) reduced pericarp growth compared with the intact pericarp by 18% at 7 DAA (Fig. 1). Removal of the seeds (SPNS) at 2 DAA resulted in slowing of pericarp growth and subsequent abscission. Treatment with GA3 or 4-Cl-IAA (50 μm) stimulated growth of deseeded pericarps compared with the deseeded control (SPNS). Application of GA3 plus 4-Cl-IAA had additive effects on growth of deseeded pericarps, resulting in growth similar to that of pericarp with seeds (SP; Fig. 1).
Figure 1.
Effect of seeds and hormones on pericarp growth. A, Effects of pericarp splitting (SP), seed removal (SPNS), and treatment of SPNS with GA3 (GA), 4-Cl-IAA (4-Cl), and GA3 plus 4-Cl-IAA (GA+4-C1) on pea pericarp growth. Pericarps at 2 DAA were split (SP) or split and deseeded. GA3 and/or 4-C1-IAA or 0.1% (v/v) Tween 80 (SP, SPNS) were applied immediately after deseeding and daily thereafter (50 μm; 30 μL at 2 and 3 DAA; 40 μL at 4, 5, and 6 DAA). Data are means ± se (n = 5). B, Representative pericarps harvested at 7 DAA are shown in picture.
Histone H2A and γ-TIP Gene Expression
Histone H2A and γ-TIP gene expression was investigated during flowering and early fruit development in pea pericarp by RNA gel-blot analysis (Fig. 2). The expression of histone H2A in the pericarp was the highest from −2 to 2 DAA and then declined rapidly. At 5 DAA, H2A transcript levels were only 2% of the levels at 2 DAA (Fig. 2B). Expression of γ-TIP was low during the early stages of fruit development (−2 to 0 DAA), increased at 1 DAA, reached maximum levels at 3 to 4 DAA, and then decreased to levels similar to prepollinated fruit (−2 DAA) by 7 DAA (Fig. 2). The maximum increase in γ-TIP mRNA levels (between 1 and 2 DAA) preceded the maximal rate of pericarp elongation (Fig. 2B).
Figure 2.
Abundance of pericarp histone H2A and γ-TIP mRNA during early pea fruit development. A, The development of early pea fruit from −2 to 7 DAA and the corresponding mRNA profiles of histone H2A and γ-TIP in the pericarp tissue. Flower bud and flower shown in picture are at −2 and 0 DAA, respectively. B, Percent relative mRNA abundance of histone H2A and γ-TIP from −2 to 7 DAA in pea pericarps and the corresponding growth rate of pea pericarps (length). Hybridization signals were analyzed by scanning autoradiograms with an imaging densitometer and normalized to the value for pericarps at 2 DAA. The relative mRNA abundance data are means ± se (n = 2), and growth rate data are means ± se (n = 5).
The SP treatment exhibited a pattern of H2A expression similar to the intact treatment (2–7 DAA; Figs. 2 and 3). Seed removal (SPNS) accelerated the decline in H2A mRNA levels after 3 DAA (Fig. 3). In general, deseeded pericarp treated with GA3, 4-C1-IAA, or GA3 plus 4-Cl-IAA (daily hormone application from 2 to 6 DAA) maintained H2A mRNA levels similar to levels detected in pericarp with seeds (Fig. 3) with one exception. At 7 DAA, all hormone-treated deseeded pericarps exhibited higher H2A mRNA levels than pericarps with seeds.
Figure 3.
Seed-specific and hormone-induced effects on pericarp histone H2A mRNA. A, RNA gel-blot analysis of histone H2A in pericarps with seeds (SP), deseeded pericarps (SPNS), and deseeded pericarps treated with GA3 (GA), 4-C1-IAA (4-Cl), and GA3 plus 4-Cl-IAA (GA+4-Cl) from 2 to 7 DAA. Pericarps at 2 DAA were split (SP) or split and deseeded. The initial hormone treatments were applied immediately after deseeding and daily thereafter as described in Figure 1. Pericarps were harvested at the indicated times 24 h after the last hormone treatment. B, Percent relative abundance of H2A mRNA. Hybridization signals were analyzed by scanning autoradiograms with an imaging densitometer and normalized to the value for pericarps at 2 DAA. Data are means ± se (n = 2).
Pericarp γ-TIP mRNA levels in the SP treatment were similar to levels in the intact treatment (2–7 DAA; Figs. 2 and 4). Seed removal (SPNS) dramatically decreased γ-TIP expression (86%) compared with SP after 3 DAA (Fig. 4). When deseeded pericarps received a daily application of 4-Cl-IAA, the pattern of γ-TIP mRNA from 3 to 7 DAA was similar to that of pericarps with seeds (SP; Fig. 4). γ-TIP mRNA levels in GA3-treated deseeded pericarps were similar to SP from 3 to 5 DAA. However, in the later stages of pericarp development (6 and 7 DAA), GA3-treated deseeded pericarps maintained elevated γ-TIP mRNA levels (Fig. 4) when the rate of pericarp growth was low (Fig. 1), in contrast to the SP and 4-Cl-IAA-treated deseeded pericarps (85%, 28%, and 21% relative mRNA abundance at 7 DAA, respectively). γ-TIP expression in GA3 plus 4-C1-IAA-treated deseeded pericarps was similar to deseeded pericarps treated with GA3.
Figure 4.
Seed-specific and hormone-induced effects on pericarp γ-TIP mRNA. RNA gel-blot analysis (A) and percent relative mRNA abundance (B) of γ-TIP in pericarps with seeds (SP), deseeded pericarps (SPNS), and deseeded pericarps treated with GA3 (GA), 4-Cl-IAA (4-Cl), and GA3 plus 4-Cl-IAA (GA+4-Cl) from 2 to 7 DAA. Pericarp treatment, harvesting, and quantitation of mRNA levels were performed as described in Figure 3. Data are means ± se (n = 2).
Structural Studies of Hormone-Treated Pea Pericarp
To attribute biological relevance to the expression patterns of H2A and γ-TIP genes and to investigate the structural changes associated with GA3 and/or 4-Cl-IAA induced pericarp growth, histological studies were undertaken. The pea pericarp consists of three distinct tissue layers: exocarp, mesocarp, and endocarp (Figs. 5 [in transverse plane] and 6 [in longitudinal plane]). At anthesis (0 DAA), the exocarp is comprised of a uniseriate epidermis, the mesocarp is composed of approximately 15 layers of vacuolated parenchyma cells, and the endocarp is composed of several layers of small undifferentiated cells (Fig. 5A). In the transverse plane (pericarp wall thickness), the endocarp middle zone parenchyma and the vascular tissues were the major zones in which cell division and differentiation occurred from 0 to 7 DAA, with little to no increase in cell number or cell layers in the mesocarp during this period (Fig. 5, A–C). The endocarp middle zone had four layers of cells at 2 DAA (Fig. 5B) that increased to five to six layers by 4 DAA. By 7 DAA the endocarp was composed of four distinct layers: an inner epidermis, a midregion of five to six layers of thin walled parenchyma, an inner layer of sclerenchyma two to three cells thick, and a transition layer lining the mesocarp (Fig. 5C). Differentiation of 7 DAA endocarp layers from deseeded pericarps treated with GA3 and/or 4-Cl-IAA was similar to pericarps with seeds (Figs. 5, D–G, and 6, D–H).
Figure 5.
Light micrographs of transverse sections of the mid-region of intact pea pericarps at 0 (A), 2 (B), and 7 DAA (C); and of 7 DAA (5 d after initial treatment) pericarps with seeds (SP; D), and SPNS treated with GA3 (E), 4-Cl-IAA (F), and GA3 plus 4-Cl-IAA (G). Sections were 2 μm thick and stained with toluidine blue. IE, Inner epidermis; P, middle zone parenchyma; S, sclerenchyma layer; TL, transition layer; and V, vascular bundles. Pericarps were treated as described in Figure 1.
Cell division was highest from 0 to 2 DAA, as indicated by increases in cell number in the longitudinal plane of the exocarp, mesocarp, the transition layer and inner epidermis of the endocarp (Fig. 6I), and the endocarp and vascular tissues in the transversve plane (Fig. 5, A and B) and by the high histone H2A mRNA levels (Fig. 2). Histone H2A mRNA levels gradually decreased from 2 to 4 DAA, a period where only the transition layer and inner epidermis layers of the endocarp increased in cell number in the longitudinal plane (Fig. 6I) and the endocarp middle zone layers increased in the transverse sections. From 4 to 7 DAA, the only increases in cell number occurred in cell layers of the endocarp middle zone layer (see increase in cell layers of the endocarp middle zone cell layer in plane perpendicular to the scale bar from 4 [Fig. 6A] to 7 DAA [Fig. 6D]); correspondingly, pericarp histone H2A mRNA levels (Fig. 2) decreased to 2% of the 2 DAA levels. The reduction and likely completion of the active cellular division phase occurred by 7 DAA in the pericarp tissue.
Figure 6.
Light micrographs of longitudinal sections of the mid-region of pea pericarps at 4 DAA: intact (A), SP (B), and deseeded pericarp (SPNS, C); and 7 DAA (5 d after initial treatment) pericarps: intact (D), SP (E), and SPNS treated with GA3 (F), 4-Cl-IAA (G), and GA3 plus 4-Cl-IAA (H). Pericarps were treated as described in Figure 1. Sections were 2 μm thick and stained with toluidine blue. The scale bar in Figure 6A (for A–C) and Figure 6D (for D–H) represents 100 μm. I, The increase in cell number in the longitudinal plane of the exocarp, mesocarp, transitional layer, and inner epidermis of intact pericarps as estimated by measuring the number of cells per unit length of fruit. The data are means ± se (n = 8).
The effect of seed removal at 2 DAA on pericarp tissues was evident by 4 DAA (Fig. 6, A–C). The 4-DAA SPNS pericarp tissue was turgid, and the endocarp, mesocarp, and exocarp layers were intact but cell expansion was minimal compared with the pericarps with seeds (Fig. 6, A–C).
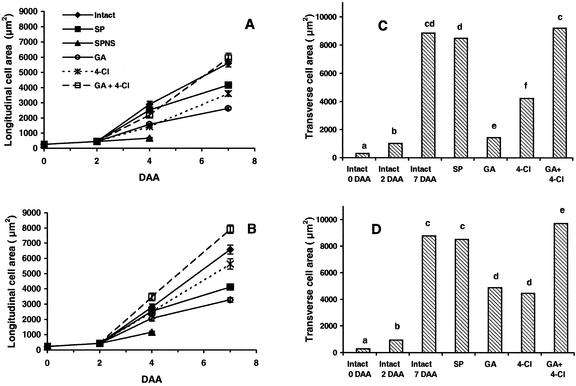
Expansion of cells occurred within the mesocarp of the intact fruits from 0 to 7 DAA (25- to 30-fold increase in longitudinal and transverse cell area), with the majority of cell growth occurring from 2 to 7 DAA (Fig. 7). Mesocarp cell elongation (in the longitudinal plane for cells proximate to the exocarp and endocarp) was greatest in intact pericarps and deseeded pericarp treated with GA3 plus 4-Cl-IAA compared with all other treatments (Fig. 7, A and B; linear interaction of intact and GA3 plus 4-Cl-IAA versus GA3, 4-Cl-IAA, and SP significant at P < 0.0001). Mesocarp cells from deseeded pericarps treated with 4-Cl-IAA expanded per unit time to a greater extent than GA3-treated deseeded pericarps (Fig. 7, A and B; linear interaction of 4-Cl-IAA versus GA3 significant at P < 0.0001). In the SPNS control, minimal mesocarp cell elongation occurred from 2 to 4 DAA (Fig. 7, A and B); SPNS pericarps usually abscise after 6 DAA.
Figure 7.
Effects of pericarp splitting (SP), seed removal (SPNS), and treatment of SPNS with GA3 (GA), 4-Cl-IAA (4-Cl), and GA3 plus 4-Cl-IAA (GA+4-Cl) on mesocarp cell size. Pericarps were treated as described in Figure 1. Mesocarp cell size from longitudinal-sectioned pericarps proximate to the exocarp (A) and proximate to the endocarp (B) was determined at 0, 2, 4, and 7 DAA (data are means ± se; n = 6). Mesocarp cell size from transverse-sectioned pericarps proximate to the exocarp (C) and proximate to the endocarp (D) was determined at 0, 2, and 7 DAA for intact pericarps and at 7 DAA (5 d after initial treatment) for SP and hormone-treated pericarps (mean separation among treatments within mesocarp regions [a–f] by lsd; P < 0.05; n = 120).
Mesocarp cells of intact, SP, and GA3 plus 4-Cl-IAA-treated deseeded pericarps (7 DAA) had significantly greater transverse-sectional area than pericarps treated with GA3 or 4-Cl-IAA alone (lsd, P < 0.05; Fig. 7, C and D). The transverse-sectional area of mesocarp cells proximate to the endocarp was significantly larger than mesocarp cells proximate to the exocarp in GA3-treated (3.3-fold greater) and GA3 plus 4-Cl-IAA-treated (1.1-fold greater) deseeded pericarps (lsd, P < 0.05). The transverse-sectional mesocarp cell size was more homogenous within the 4-Cl-IAA treatment. To determine whether the observed heterogeneity in transverse-sectional mesocarp cell size with GA3-treated pericarp was an application effect, GA3 was applied to the exocarp of pericarps in selected treatments. Application of GA3 to the exocarp also resulted in larger mesocarp cells in the layers proximal to the endocarp (transverse sections; data not shown). Substantial trichome development from the inner epidermis of the endocarp was observed in pericarp with seeds (SP) and the hormone-treated deseeded pericarps (Figs. 5, D–G, and 6, E–H).
DISCUSSION
The current work employed molecular and histological methods to discriminate how seeds and naturally occurring pea hormones regulate cell division and elongation in pea fruit (−2 to 7 DAA). The expression pattern of the γ-TIP and histone H2A genes in pea pericarp indicated that cell division and elongation phases overlap in early pericarp development (Fig. 2). Mitotic activity was developmentally regulated within each pericarp tissue layer in both the longitudinal and transverse planes (Figs. 5, A–C, and 6I) and closely followed the expression pattern of histone H2A in pericarp from −2 to 7 DAA (Fig. 2). Therefore, histone H2A in pea fruit is replication dependent, and accumulation of its mRNA is a useful marker for cell division in this tissue. γ-TIP mRNA levels were also developmentally regulated in pea pericarp. A 2-fold increase in γ-TIP mRNA levels in intact pericarps (from 0 to 2 DAA; Fig. 2) preceded the peak in pericarp growth rate (4 DAA; Fig. 2) and rapid mesocarp cell expansion (in the longitudinal plane; 2–7 DAA; Fig. 7). After the peak in pericarp growth rate (length) and γ-TIP mRNA levels (4 DAA), γ-TIP message decreased in parallel with the reduction in the pericarp growth rate (5–7 DAA; Fig. 2). However, mesocarp cells continued to expand at a similar rate from 4 to 7 DAA (intact treatment; Fig. 7, A and B). These data suggest that the majority of γ-TIP message is required during the onset and mid-phase of rapid cellular expansion and that γ-TIP message is not limiting for continued cell expansion after this period. γ-TIP mRNA levels from the SP treatment were similar in abundance and pattern to that of the intact pericarps from 2 to 7 DAA (Figs. 2 and 4). However, the rate of SP mesocarp cell expansion differed from intact pericarps (SP rate was greater from 2 to 4 DAA than from 4 to 7 DAA), and the mesocarp cell size at 7 DAA in SP pericarps was significantly smaller than intact pericarps (Fig. 7, A and B). In Arabidopsis, γ-TIP mRNA was observed to be primarily expressed at the time when large central vacuoles are being formed during cell enlargement (root cell elongation zone and shoot tissues from elongating seedlings; Ludevid et al., 1992). The data in this study are in agreement with the observations of Ludevid et al. (1992) that γ-TIP message, in general, is a marker of expanding tissue. However, our data also suggest that although γ-TIP message is a qualitative marker of expanding or elongating tissue, it is not a quantitative marker for cell expansion.
GAs and auxins have been shown to regulate cell division and cell enlargement in a number of plant systems (Bayliss, 1985; Behringer et al., 1990; Sauter and Kende, 1992; Cleland, 1995). However, because the method of application, tissue treatment, and species studied for auxin-stimulated growth and GA-stimulated growth usually vary, direct comparisons of the actions of these hormones on growth processes are rarely studied. Using the split-pericarp system, we studied the effects of seeds and hormones on growth and development of the pea pericarp, a tissue responsive (with respect to growth) to both GAs and auxin (4-Cl-IAA). Removal of seeds caused a rapid decrease in growth (Fig. 1) and a reduction in abundance of the histone H2A and γ-TIP mRNA levels (Figs. 3 and 4) in the pericarp. Daily treatment of deseeded pericarps with 4-Cl-IAA and/or GA3 stimulated pericarp growth and maintained H2A gene expression and cellular development similar to pericarps with seeds (Figs. 1, 3, 5, D–G, and 6, E–H). Vercher et al. (1984) and Vercher and Carbonell (1991) also found that GA3 or 2,4-dichlorophenoxyacetic acid could maintain endocarp development (transverse plane) in non-pollinated ovaries of pea. These data suggest that hormones play an important role in maintaining cellular division and maturation in the pericarp. However, since GA3 and 4-Cl-IAA alone, and in combination, affect cell division in postanthesis pericarp similarly, the differences observed in growth between the GA3 plus 4-Cl-IAA-treated deseeded pericarp and deseeded pericarp treated with GA3 or 4-Cl-IAA only are likely due to cell enlargement.
In 4-Cl-IAA-treated deseeded pericarps, pericarp growth rate (length) trend (Fig. 1), γ-TIP mRNA abundance pattern (Fig. 4), and cellular expansion trend (Fig. 7, A and B) were similar to that in pericarps with seeds (SP). γ-TIP mRNA levels in GA3-treated deseeded pericarps were maintained at levels equivalent to SP from 3 to 5 DAA (Fig. 4). Phillips and Huttly (1994) also found that γ-TIP mRNA abundance increased in the Arabidopsis ga1 mutant 24 h after GA application (24 h before detectable extension growth); one application of GA3 increased the γ-TIP mRNA levels for at least 72 h (composite sample of flower stems and buds). However, daily application of GA3 to deseeded pericarps resulted in maintenance of high γ-TIP mRNA levels at 6 and 7 DAA (Fig. 4), a time when the rate of pericarp elongation was low (Fig. 1). Maintenance of high γ-TIP mRNA levels in the GA3-treated deseeded pericarps from 4 to 7 DAA did not result in larger pericarps (length; Fig. 1) or larger mesocarp cells (Fig. 7, A and B) when compared with other treatments, suggesting γ-TIP message was not limiting for cell growth at this stage.
These data are consistent with the hypothesis that cell expansion induced by GA and/or auxin results in stress relaxation of the cell wall and a need for water uptake (Behringer et al., 1990; Cleland, 1995). This likely requires an increase in the hydraulic conductance of the tonoplast membrane that, in turn, requires increased γ-TIP expression. Therefore, in general, high γ-TIP mRNA levels are associated with elongating tissue, but certain conditions/factors can alter this relationship (for example, exogenous GA).
In the transverse plane, GA3 application to deseeded pericarps promoted cell enlargement mainly in cells of the mesocarp proximal to the endocarp (Fig. 7, C and D), suggesting that stimulation of cell expansion by GA3 is cell specific within the mesocarp tissue. The spatial responsiveness to GA3 could be the consequence of changes in the levels of active GAs within these cells or differences in cellular sensitivity, but was not because of the method of hormone application. Vercher and Carbonell (1991) did not observe differential cell enlargement in the mesocarp of GA3-treated unpollinated fruits at 5 DAA. The differences observed between our studies and Vercher and Carbonell's may be due to the type of tissue used and the timing of the GA3 treatments (application of GA3 to unpollinated ovaries at 0 DAA by Vercher and Carbonell [1991] versus application of GA3 to pollinated deseeded ovaries at 2 DAA in our studies).
In summary, pollination stimulates pea pericarp development, which involves a balance of cell division, elongation, and differentiation. The pattern of cell division and enlargement in pea pericarp is disrupted when seeds are removed at 2 DAA, indicating that sustenance of these processes in pea fruit requires the presence of the seeds. GA and 4-Cl-IAA maintained these processes in deseeded pericarps similarly to pericarps with seeds, and both hormones were required to obtain mesocarp cell sizes equivalent to intact fruit. The novel use of histone H2A and γ-TIP gene expression as molecular markers of cell division and elongation during fruit development, respectively, has aided our understanding of cellular processes when used in conjunction with histological studies. Histone H2A was replication dependent and useful as a marker for cell division during early pericarp growth. γ-TIP message was a qualitative marker of expanding or elongating tissue, but was not a quantitative marker for cell expansion. Our data support the theory that cell division and elongation are developmentally regulated by the developing seeds during early pea fruit growth and are maintained by hormonal interaction of GA and auxin.
MATERIALS AND METHODS
Plant Material and Treatments
Plants of pea (Pisum sativum) line I3 (Alaska-type) were grown as previously described (van Huizen et al., 1995). One fruit per plant (at the third to fifth flowering node) was used per treatment, and subsequent flowers were removed as they developed. Terminal apical meristems of plants were intact, and the fruit or treated pericarp remained attached to the plant during the entire experiment. To remove the seeds, a split-pericarp technique was used as described by Ozga et al. (1992). In brief, pericarps of 2 DAA (15- to 22-mm) ovaries (pericarp plus seeds) were left intact (intact treatment) or split down the dorsal suture, either without disturbing the seeds (SP treatment) or with the seeds removed immediately (SPNS treatment). GA3 and/or 4-Cl-IAA (50 μm in 0.1% [v/v] aqueous Tween 80) were applied immediately after deseeding to the inner pericarp wall (endocarp) and daily thereafter to 6 DAA (30 μL, 2 and 3 DAA; and 40 μL, 4–6 DAA). Control treatments (SP and SPNS) were treated with 0.1% (v/v) aqueous Tween 80. High humidity was maintained by enclosing the pericarps in clear plastic bags throughout the duration of the experiment.
RNA Isolation and Northern-Blot Analysis
For each sample, two to three pods were ground in liquid N2 and a 0.3- to 0.5-g subsample was used for RNA extraction. Total RNA was extracted following the method of Chomczynski and Sacchi (1987) with two additional chloroform extractions after the first chloroform extraction to remove polysaccharides. For northern analysis, the total RNA samples (10 μg) were denatured in 2.2 m formaldehyde/50% (v/v) formamide, fractionated on a 1.2% (w/v) agarose/2.2 m formaldehyde gel using a 20 mm MOPS buffer (pH 7.0; Maniatis et al., 1982), and transferred to Nitroplus membranes (MSI, Westborough, MA) with 10× SSC. Equal loading and RNA integrity were ascertained by ethidium bromide staining of rRNA bands before transfer. Membranes were baked for 2 h at 80°C under a vacuum.
The [32P]dATP random-primed cDNA probes were synthesized using the random primers DNA labeling system (Invitrogen, Carlsbad, CA) according to the manufacturer's instructions. Membranes were prehybridized and then hybridized with the labeled probe at 65°C in a solution containing 6× SSPE, 0.5% (w/v) SDS, 5× Denhardt's solution (1% [w/v] Ficoll 400, 1% [w/v] polyvinylpyrrolidone, 1% [w/v] bovine serum albumin), and 100 μg mL−1 tRNA for 18 h. Membranes were washed three times for 20 min at room temperature in 2× SSPE and 0.1% (w/v) SDS, and once in 0.1% (v/v) SSC and 0.1% (w/v) SDS at 65°C, and placed at −70°C with X-Omat AR film (Eastman-Kodak, Rochester, NY). The northerns were probed with a 0.6-kb EcoRI fragment of histone H2A cDNA from pea (Koning et al., 1991) and a 1.4-kb BamHI-HindIII fragment of γ-TIP cDNA from Arabidopsis (Höfte et al., 1992). The amount of labeled antisense RNA hybridization to the RNA blot was quantitated by scanning the autoradiogram with an imaging densitometer (Bio-Rad, Hercules, CA; van Huizen et al., 1997).
For quantitation of transcript levels during early fruit development, one extraction of 15 pericarps of the 2 DAA treatment was performed, and this sample was run on all gels as a quantitative standard. The value for histone H2A and γ-TIP signals at 2 DAA on each autoradiogram was designated 100%, and all other signals were calculated relative to that sample.
Light Microscopy
Light microscopy was conducted on transverse and longitudinal sections of the midregion of the pericarp wall. Fixation was overnight at 20°C in 3% (v/v) glutaraldehyde fixative in 0.1 m phosphate buffer (pH 6.8). After fixation, tissue segments were dehydrated through a graded series of ethanol (at 30-min intervals for each 15% increment of ethanol) and embedded in Spurr's resin (Spurr, 1969). Embedded tissues were sectioned 2 μm thick using a glass knife and an ultramicrotome (Om U 2, Reichert, Vienna) and stained with 0.5% (w/v) toluidine Blue-O in 0.1% (w/v) sodium carbonate (pH 11.1).
Mesocarp cell area was determined in two regions of mesocarp tissue (cells proximate to the endocarp and to the exocarp) avoiding vascular bundles. For the transverse sections, 10 adjacent cells for each region per section, three sections per fruit, and four fruit per treatment were measured at 0, 2, and 7 DAA (5 d after initial hormone treatments). To compare the linear increase in cell size among the treatments in the longitudinal plane, a second set of fruits was treated, sectioned longitudinally, and cell area-quantitated at 0, 2, 4, and 7 DAA. Longitudinal cell area was determined by measuring two 10-cell areas for each region per section, three sections per fruit, and two fruit per treatment. The cells were viewed through a compound microscope at 10× or 25× objective lens magnification, the image was relayed through a video camera (Color Video Camera/CCD-IRIS, Sony, Tokyo) to an attached Magnavox computer monitor, and the size of mesocarp cells was determined using image analysis software (Northern Exposure 2.9×, Empix Imaging Inc., Mississauga, ON, Canada).
The increase in cell number in the transverse plane (thickness of pericarp wall) was estimated by counting the number of cells layers in the transverse fruit sections. The increase in cell number in the longitudinal plane was estimated by measuring the number of cells per unit length (at 10×, 20×, or 40× objective lens magnification, as required) for each tissue (exocarp, mesocarp [between the mesocarp vascular bundles and exocarp layer], transition layer, and inner epidermis of the endocarp) per section, four sections per fruit, and two fruit per treatment. The number of cells per length of fruit was calculated as follows:
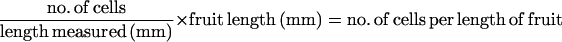 |
1 |
Statistical Analysis
The cell area data were analyzed using an analysis of variance test following a completely randomized design for the cell area of transverse sections and a 2-factor factorial design for the cell area of longitudinal sections. The longitudinal cell area data were tested for linear trends and interactions.
ACKNOWLEDGMENTS
The authors would like to acknowledge the following for their technical assistance on this work: Roisin McGarry for her statistical analysis of data, Maryse Maurice for fixing and sectioning of some tissues, and Daman Vig for cell area quantitation.
Footnotes
This research was supported by the National Sciences and Engineering Research Council of Canada (award no. OGP0138166).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.010800.
LITERATURE CITED
- Bayliss MW. Regulation of the cell division cycle in cultured plant cells. In: Bryant JA, Francis D, editors. The Cell Division Cycle in Plants. Cambridge: Cambridge University Press; 1985. pp. 157–177. [Google Scholar]
- Behringer FJ, Cosgrove DJ, Reid JB, Davies PJ. Physical basis for altered stem elongation rates in internode length mutants of Pisum. Plant Physiol. 1990;94:166–173. doi: 10.1104/pp.94.1.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Cleland RE. Auxin and cell elongation. In: Davies PJ, editor. Plant Hormones: Physiology, Biochemistry and Molecular Biology. Ed 2. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1995. pp. 214–227. [Google Scholar]
- Davies PJ. The plant hormones: their nature, occurrence, and functions. In: Davies PJ, editor. Plant Hormones, Physiology, Biochemistry and Molecular Biology. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1995. pp. 1–12. [Google Scholar]
- Garcia-Martinez JL, Santes C, Croker SJ, Hedden P. Identification, quantitation and distribution of gibberellins in fruits of Pisum sativum L. cv. Alaska during pod development. Planta. 1991;184:53–60. doi: 10.1007/BF00208236. [DOI] [PubMed] [Google Scholar]
- Hebblethwaite PD, Heath MC, Dawkins TCK. The Pea Crop: A Basis for Improvement. London: Butterworths; 1985. [Google Scholar]
- Höfte H, Hubbard L, Reizer J, Ludevid D, Herman EM, Chrispeels MJ. Vegetative and seed-specific forms of tonoplast intrinsic protein in the vacuolar membrane of Arabidopsis thaliana. Plant Physiol. 1992;99:561–570. doi: 10.1104/pp.99.2.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koning AJ, Tanimoto EY, Kiehne K, Rost T, Comai L. Cell-specific expression of plant histone H2A genes. Plant Cell. 1991;3:657–665. doi: 10.1105/tpc.3.7.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockhart JA. An analysis of irreversible plant cell elongation. J Theor Biol. 1965;8:264–275. doi: 10.1016/0022-5193(65)90077-9. [DOI] [PubMed] [Google Scholar]
- Ludevid D, Höfte H, Himelblau E, Chrispeels MJ. The expression pattern of the tonoplast intrinsic protein γ-TIP in Arabidopsis thaliana is correlated with cell enlargement. Plant Physiol. 1992;100:1633–1639. doi: 10.1104/pp.100.4.1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKenzie-Hose AK, Ross JJ, Davies NW, Swain SM. Expression of gibberellin mutations in fruits of Pisum sativum L. Planta. 1998;204:397–403. [Google Scholar]
- Magnus V, Ozga JA, Reinecke DM, Pierson GL, Larue TA, Cohen JD, Brenner ML. 4-Chloroindole-3-acetic and indole-3-acetic acids in Pisum sativum. Phytochemistry. 1997;46:675–681. [Google Scholar]
- Maniatis T, Fritsch EF, Sambrook J. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1982. pp. 202–203. [Google Scholar]
- Marumo S, Hattori H, Abe H, Munakata K. Isolation of 4-chloroindolyl-3-acetic acid from immature seeds of Pisum sativum. Nature. 1968;219:959–960. doi: 10.1038/219959b0. [DOI] [PubMed] [Google Scholar]
- Maurel C. Aquaporins and water permeability of plant membranes. Annu Rev Plant Physiol Plant Mol Biol. 1997;48:399–429. doi: 10.1146/annurev.arplant.48.1.399. [DOI] [PubMed] [Google Scholar]
- Maurel C, Reizer J, Schroeder JI, Chrispeels MJ. The vacuolar membrane protein γ-TIP creates water specific channels in Xenopus oocytes. EMBO J. 1993;12:2241–2247. doi: 10.1002/j.1460-2075.1993.tb05877.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müntz K, Rudolph A, Schlesier G, Silhengst P. The function of the pericarp in fruits of crop legumes. Kulturpflanze. 1978;26:37–67. [Google Scholar]
- Osley MA. The regulation of histone synthesis in the cell cycle. Annu Rev Biochem. 1991;60:827–861. doi: 10.1146/annurev.bi.60.070191.004143. [DOI] [PubMed] [Google Scholar]
- Ozga JA, Brenner ML, Reinecke DM. Seed effects on gibberellin metabolism in pea pericarp. Plant Physiol. 1992;100:88–94. doi: 10.1104/pp.100.1.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozga JA, Reinecke DM. Interaction of 4-chloroindole-3-acetic acid and gibberellins in early pea fruit development. Plant Growth Regul. 1999;27:33–38. [Google Scholar]
- Phillips AL, Huttly AK. Cloning of two gibberellin-regulated cDNAs from Arabidopsis thaliana by subtractive hybridization: Expression of the tonoplast water channel, γ-TIP, is increased by GA3. Plant Mol Biol. 1994;24:603–615. doi: 10.1007/BF00023557. [DOI] [PubMed] [Google Scholar]
- Reinecke DM, Ozga JA, Magnus V. Effect of halogen substitution of indole-3-acetic acid on biological activity in pea fruit. Phytochemistry. 1995;40:1361–1366. [Google Scholar]
- Rodrigo MJ, Garcia-Martinez JL, Santes CM, Gaskin P, Hedden P. The role of gibberellins A1 and A3 in fruit growth of Pisum sativum L. and the identification of gibberellins A4 and A7 in young seeds. Planta. 1997;201:446–455. [Google Scholar]
- Sauter M, Kende H. Gibberellin-induced growth and regulation of the cell division cycle in deepwater rice. Planta. 1992;188:362–368. doi: 10.1007/BF00192803. [DOI] [PubMed] [Google Scholar]
- Sponsel VM. Effects of applied gibberellins and naphthylacetic acid on pod development in fruits of Pisum sativum L. cv. Progress No. 9. J Plant Growth Regul. 1982;1:147–152. [Google Scholar]
- Spurr AR. A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res. 1969;26:31–43. doi: 10.1016/s0022-5320(69)90033-1. [DOI] [PubMed] [Google Scholar]
- Sutcliffe JF, Pate JS. The Physiology of the Garden Pea. London: Academic Press; 1977. [Google Scholar]
- Tanimoto EY, Rost TL, Comai L. DNA replication-dependent histone H2A mRNA expression in pea root tips. Plant Physiol. 1993;103:1291–1297. doi: 10.1104/pp.103.4.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Huizen R, Ozga JA, Reinecke DM. Influence of auxin and gibberellin on in vivo protein synthesis during early pea fruit growth. Plant Physiol. 1996;112:53–59. doi: 10.1104/pp.112.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Huizen R, Ozga JA, Reinecke DM. Seed and hormonal regulation of gibberellin 20-oxidase expression in pea pericarp. Plant Physiol. 1997;115:123–128. doi: 10.1104/pp.115.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Huizen R, Ozga JA, Reinecke DM, Twitchin B, Mander LN. Seed and 4-chloroindole-3-acetic acid regulation of gibberellin metabolism in pea pericarp. Plant Physiol. 1995;109:1213–1217. doi: 10.1104/pp.109.4.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vercher Y, Carbonell J. Changes in the structure of ovary tissues and in the ultrastructure of mesocarp cells during ovary senescence or fruit development induced by plant growth substances in Pisum sativum. Physiol Plant. 1991;81:518–526. [Google Scholar]
- Vercher Y, Molowny A, Lopez C, Garcia-Martinez JL, Carbonell J. Structural changes in the ovary of Pisum sativum L. induced by pollination and gibberellic acid. Plant Sci Lett. 1984;36:87–91. [Google Scholar]